搜索


Recently, the internationally renowned journal The Proceedings of the National Academy of Sciences (PNAS) published online the collaborative research findings of the Ultrafast Science Research Center at Zhangjiang Institute for Advanced Study, Shanghai Jiao Tong University, titled “Elucidation of a distinct photoreduction pathway in class II Arabidopsis thaliana photolyase”.The corresponding authors are Professors Bei Ding and Dongping Zhong from the Ultrafast Science Center.The first authors are Zhongneng Zhou, Zijing Chen, and Xiuwen Jiang from the same center.
In biological systems, DNA photolyases (PLs) and cryptochromes (Crys) play critical roles in DNA repair and light sensing, respectively.These enzymes contain a tryptophan (Trp) triad or a fourth tyrosine (Tyr) to form a photoreduction network (Figure 1).However, their quantum yield for DNA repair is lower than that of class I photolyases.To better understand the photoreduction mechanism of class II photolyases, the research team conducted a pioneering investigation.Using mutation design and femtosecond spectroscopy, they successfully elucidated a distinct photoreduction mechanism in the class II photolyase (AtPL) of Arabidopsis thaliana.This discovery not only provides new insights into the functional diversity of the photolyase family but also opens new directions for future research in related fields.
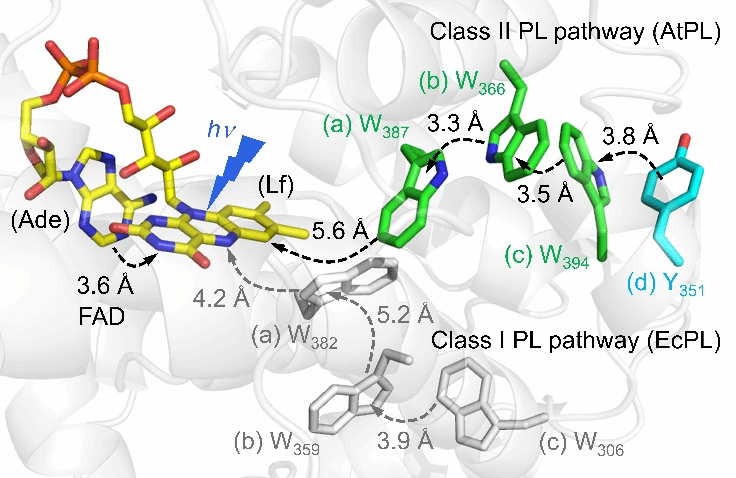
By constructing a W387F mutant to block the electron transfer chain in AtPL, the researchers found that the flavin adenine dinucleotide (FAD) cofactor in AtPL exhibits unique reverse electron transfer kinetics between flavin (Lf) and adenine (Ade).The forward electron transfer (fET) time constant was measured at 172 ps, while the reverse electron transfer (rET) occurred in just 26 ps (Figure 2)—a stark contrast to the normal kinetics observed in class I EcPL【1】.
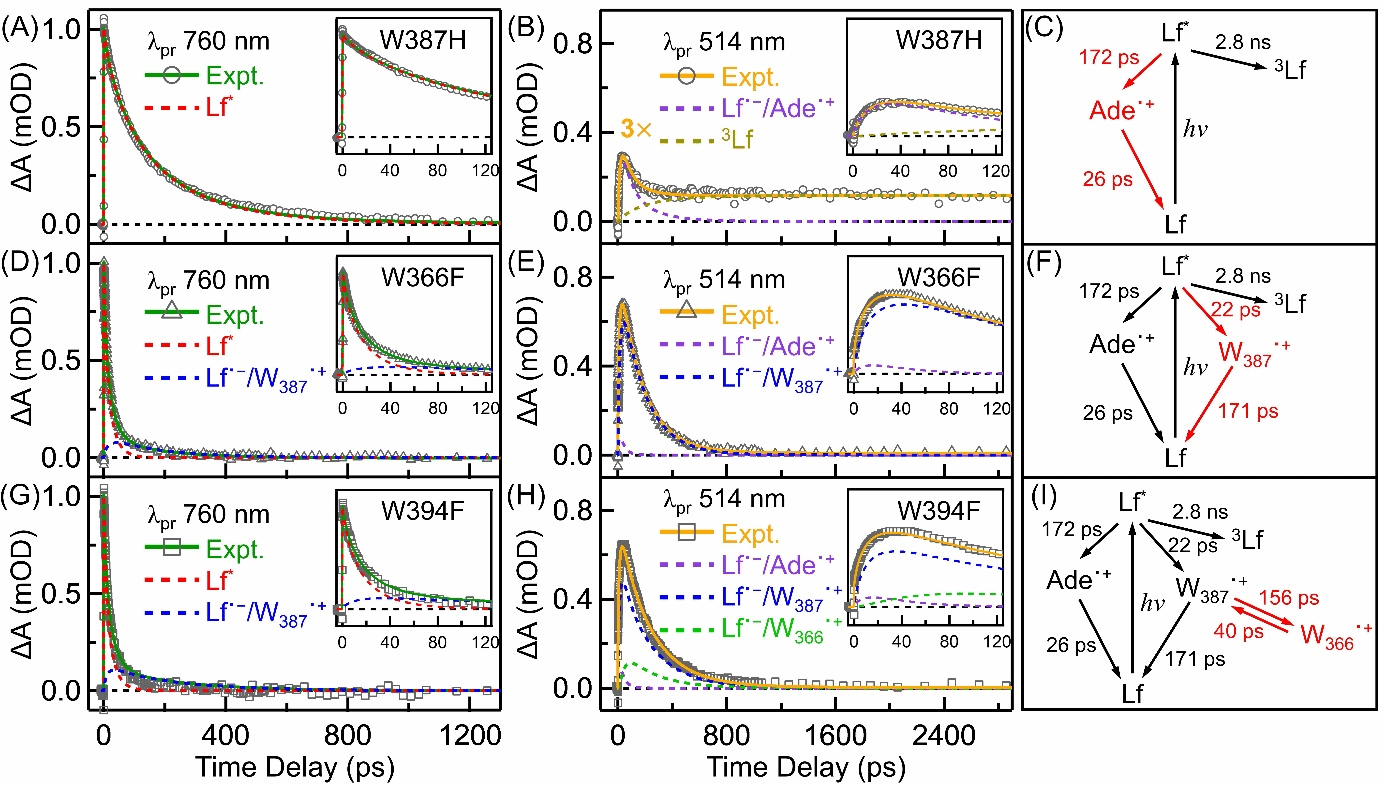
Using Marcus theory to estimate reorganization energies, they found significant differences between class I and class II photolyases, possibly due to structural differences leading to more water molecules in the FAD binding pocket of class II photolyases.The team further examined the electron transfer rate from Lf to the nearest tryptophan (Wa) in AtPL using the W366F mutant.Data analysis revealed that in the W366F mutant, the fET from W387 to Lf occurred in 22 ps, while the rET occurred in 171 ps (Figure 2).Marcus theory calculations estimated the ΔG₀ of fET to be about -0.58 eV and the reorganization energy to be 1.10 eV.Experiments with the W394F mutant showed that the electron transition from Wa to Wb is energetically unfavorable.Based on lifetimes of 156 ps (fET) and 40 ps (rET), the estimated ΔG₀ was around 0.04 eV—unlike the energetically favorable transitions observed in class I EcPL【1】.This suggests a unique electron transfer mechanism in AtPL.Such an energetically uphill ET step explains why the quantum yield of photoreduction in class II AtPL (~34%) is lower than that in class I EcPL (~45%).
By designing the Y351F mutant, the team also captured the rapid deprotonation of the distal tryptophan residue Wc in class II AtPL (Figure 3).
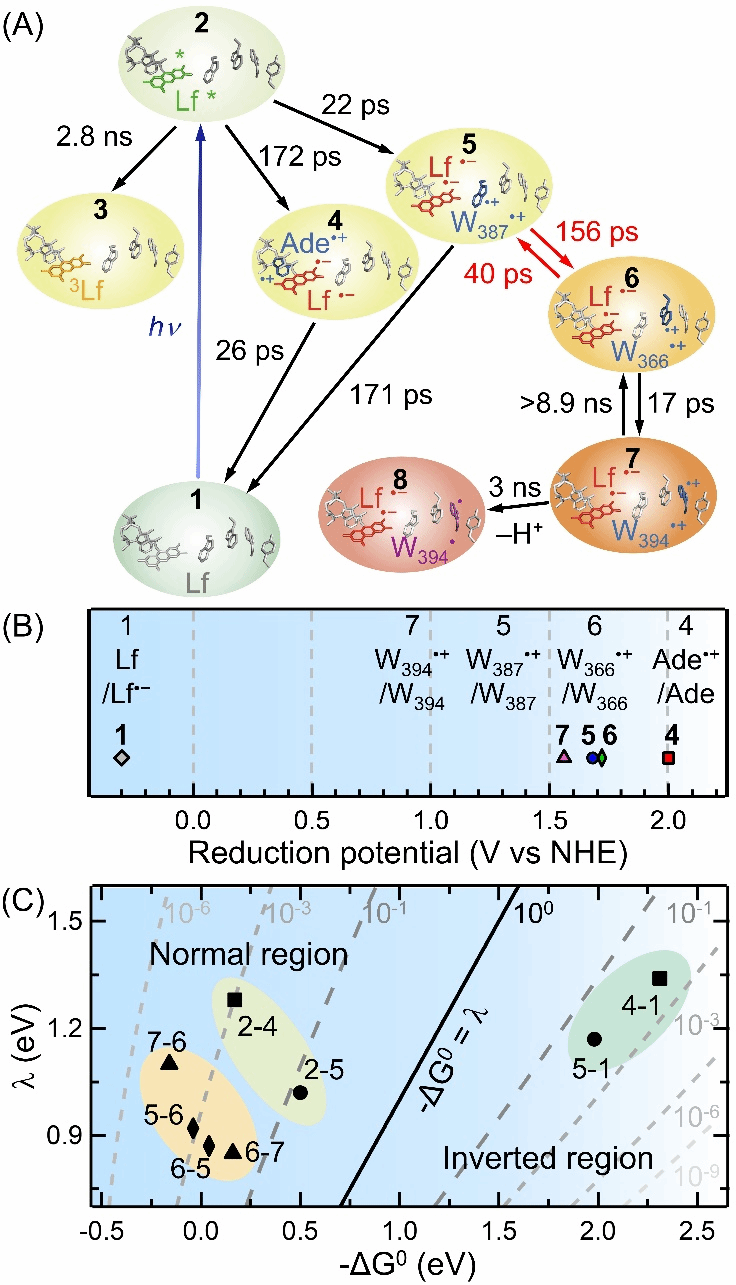
During photoreduction, Wc rapidly lost a proton within 3 ns to form a neutral radical Wc˙.This observation is consistent with the rapid Wc deprotonation reported in class II MmPL【2】.Such fast deprotonation not only highlights the unique photoreduction mechanism of class II photolyases but may also relate to protein dynamics.Its biological significance and role in photolyase evolution will be further explored.This research provides deep insight into the photoreduction mechanism of class II AtPL, aiding future designs of artificial photolyases and photoreceptor studies.Additionally, the work raises important questions about the unique photoreduction network and structural evolution of class II photolyases.
This study was supported by the Ministry of Science and Technology of China (2020YFA0509700) and the National Natural Science Foundation of China (22373067, 12004246, 22433003).
Paper link: https://www.pnas.org/doi/abs/10.1073/pnas.2416284121
References:
Z. Liu, et al. Determining complete electron flow in the cofactor photoreduction of oxidized photolyase. Proc. Natl. Acad. Sci. U. S. A. 110, 12966–12971 (2013).
P. Müller, E. Ignatz, S. Kiontke, K. Brettel, L.-O. Essen, Sub-nanosecond tryptophan radical deprotonation mediated by a protein-bound water cluster in class II DNA photolyases. Chem. Sci. 9, 1200–1212 (2018).






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn