搜索


Two-dimensional metal-organic frameworks (MOFs) have demonstrated broad prospects in fields such as energy devices, optoelectronics, and biological applications, owing to their chemical tunability, structural anisotropy, and outstanding charge transport properties. These characteristics arise from two key factors: (1) in-plane conjugated networks formed via orbital hybridization between metals and ligands, and (2) out-of-plane van der Waals interactions. However, reports on 2D MOFs based on metal–carbon coordination are rare, despite their notable potential in photoactivity and catalytic activity. The scarcity of such 2D MOFs is mainly attributed to their poor chemical stability and demanding synthesis conditions.
Metal–carbon coordination environments have long been important constructs in the development of catalysts in organic synthesis. Metal–organic complexes with metal–carbon sites play crucial roles in organic catalysis and pharmaceutical synthesis. However, such molecular catalysts often suffer from low chemical stability. Porous and stable crystalline 2D MOFs can serve as ideal carriers for metal–carbon active sites. Although researchers have attempted to introduce metal–carbon sites into 2D MOFs via post-synthetic modification, these methods often suffer from low metal loading, poor stability, and difficulties in structural characterization. The direct synthesis of 2D MOFs based on metal–carbon coordination remains a long-standing challenge, not to mention systematic studies on their electrical properties and catalytic activities. The lack of universal synthetic strategies and suboptimal crystallinity have hindered the study of structure–property relationships and the application development of this class of materials.
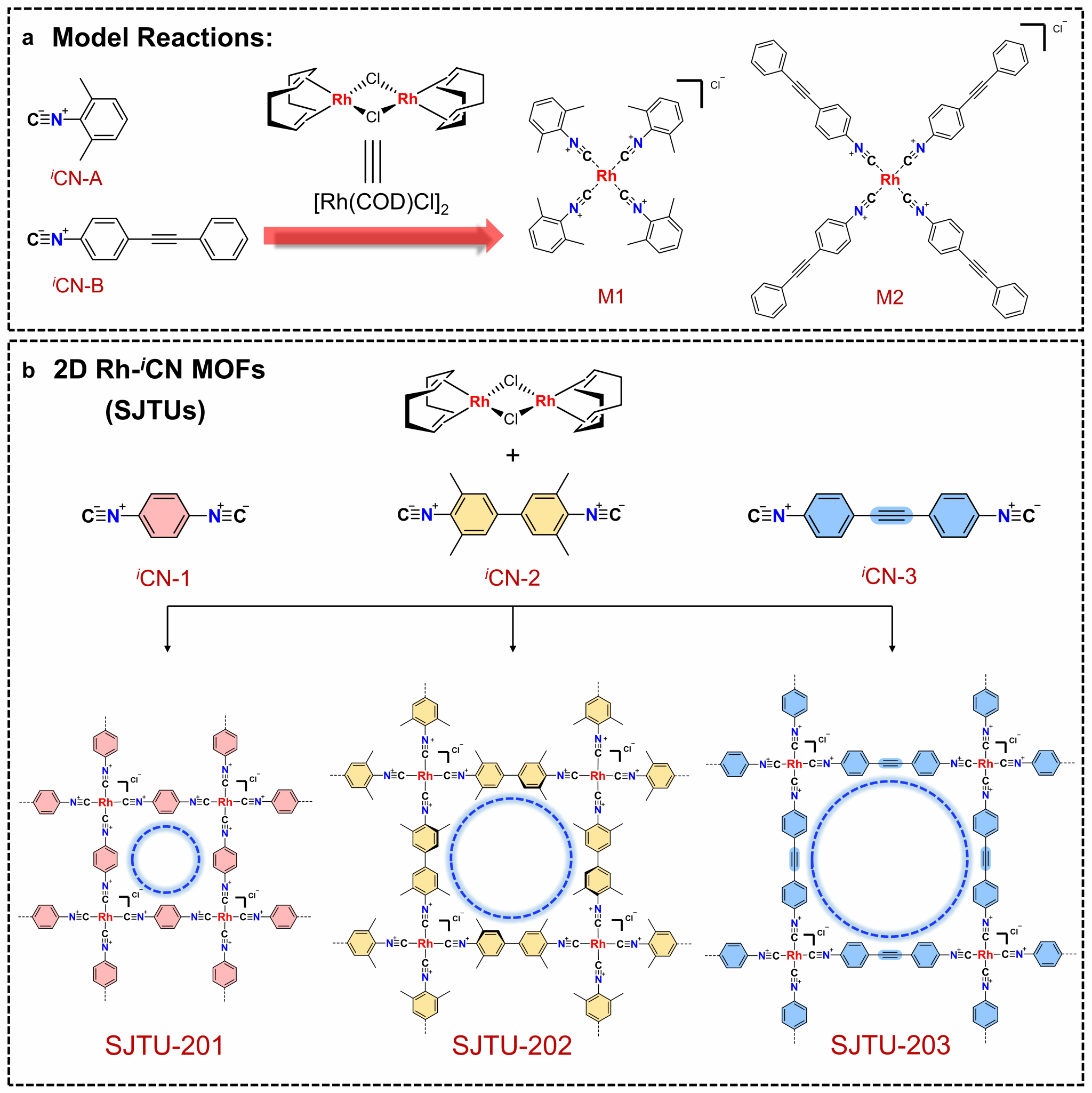
Recently, Professor Xiaodong Zhuang's team at the School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, developed a series of 2D MOFs based on rhodium–isocyanide (Rh–iCN) coordination via a simple solution synthesis under an inert atmosphere (Figure 1). This follows their December 2024 development of Ag–iCN-based 2D MOFs and represents a new 2D coordination polymer system based on metal–carbon coordination bonds (Angew. Chem. Int. Ed. 2025, 64, e202417658; doi.org/10.1002/anie.202417658).
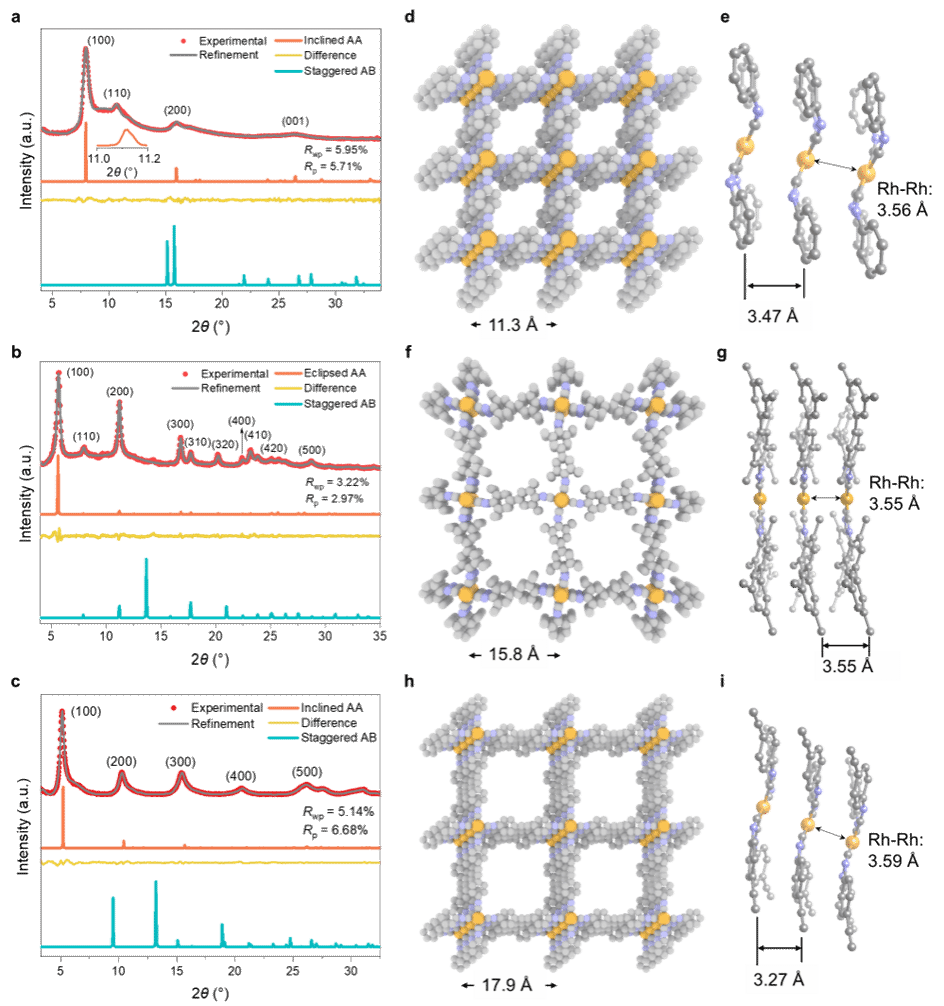
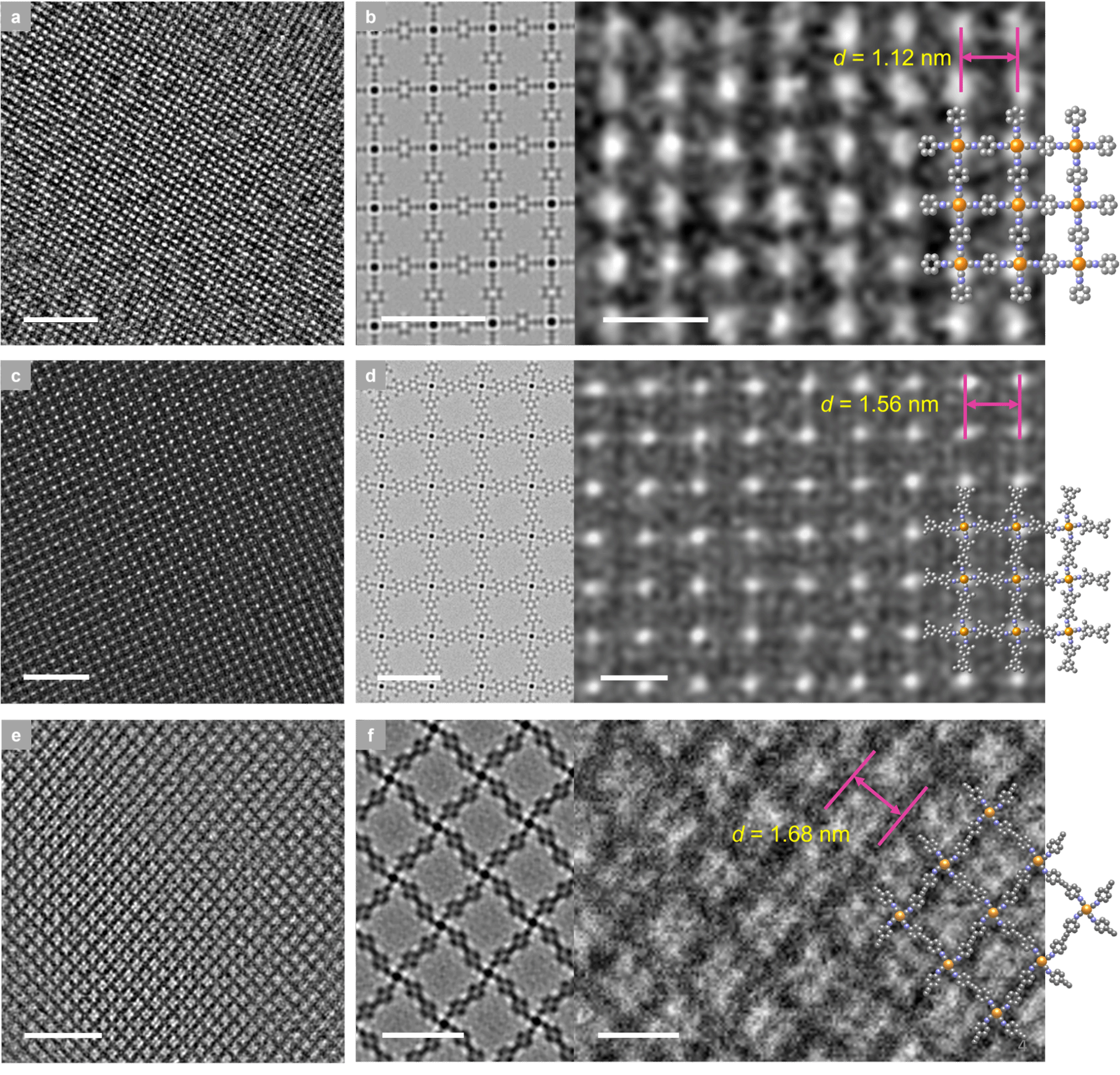
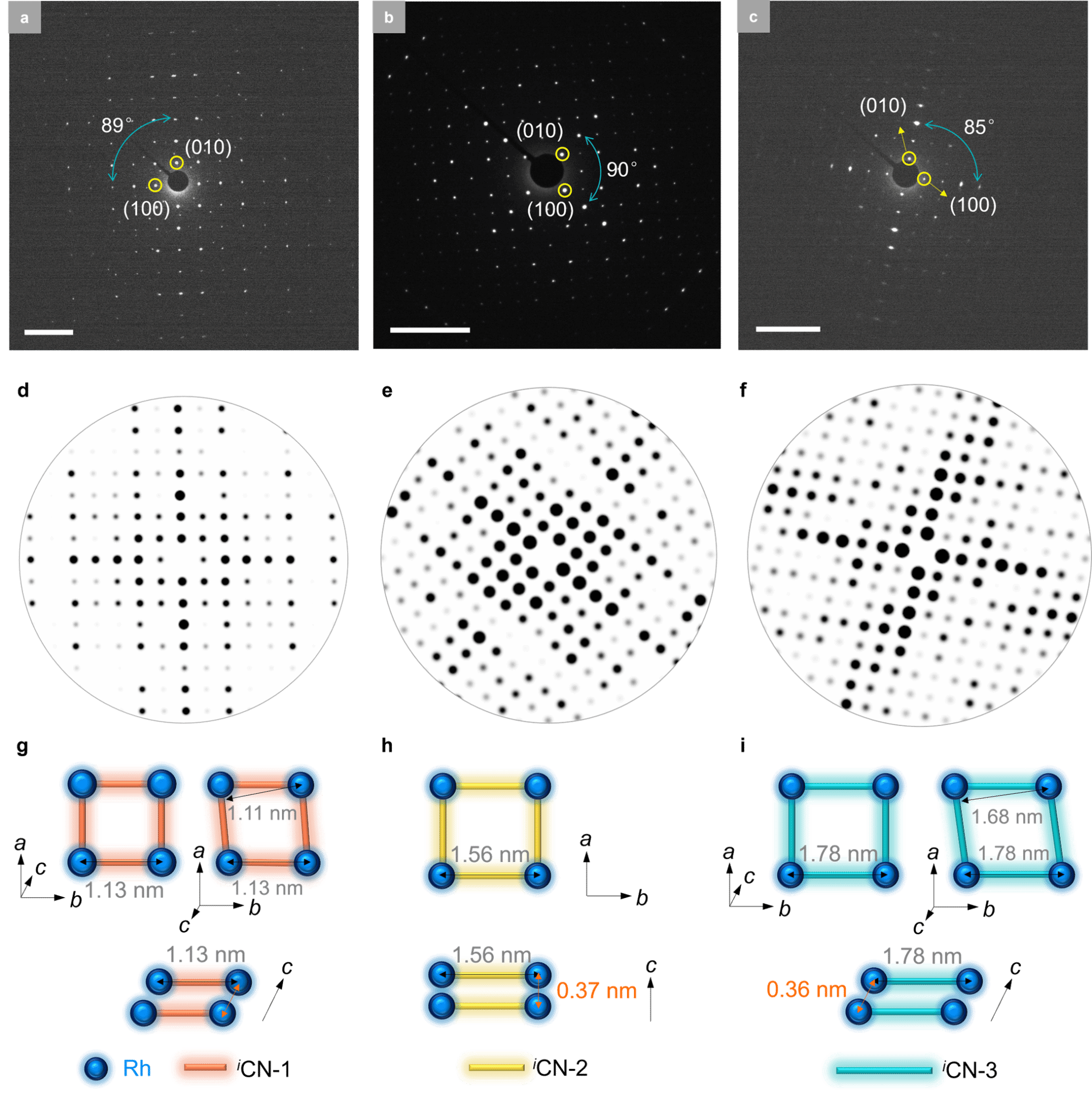
These 2D MOFs were synthesized via coordination reactions between aryl di-isocyanide ligands and chloro(1,5-cyclooctadiene)rhodium(I) dimers. Rh–C₄ coordination sites centered on Rh(I) were directly incorporated into the 2D MOF lattice, resulting in air-stable structures. Powder X-ray diffraction confirmed that the synthesized 2D MOFs possessed high crystallinity and adopted a layered AA stacking mode (Figure 2). Aberration-corrected high-resolution transmission electron microscopy and electron diffraction analysis confirmed that the 2D MOFs exhibit an in-plane quasi-square lattice network (Figures 3 and 4).
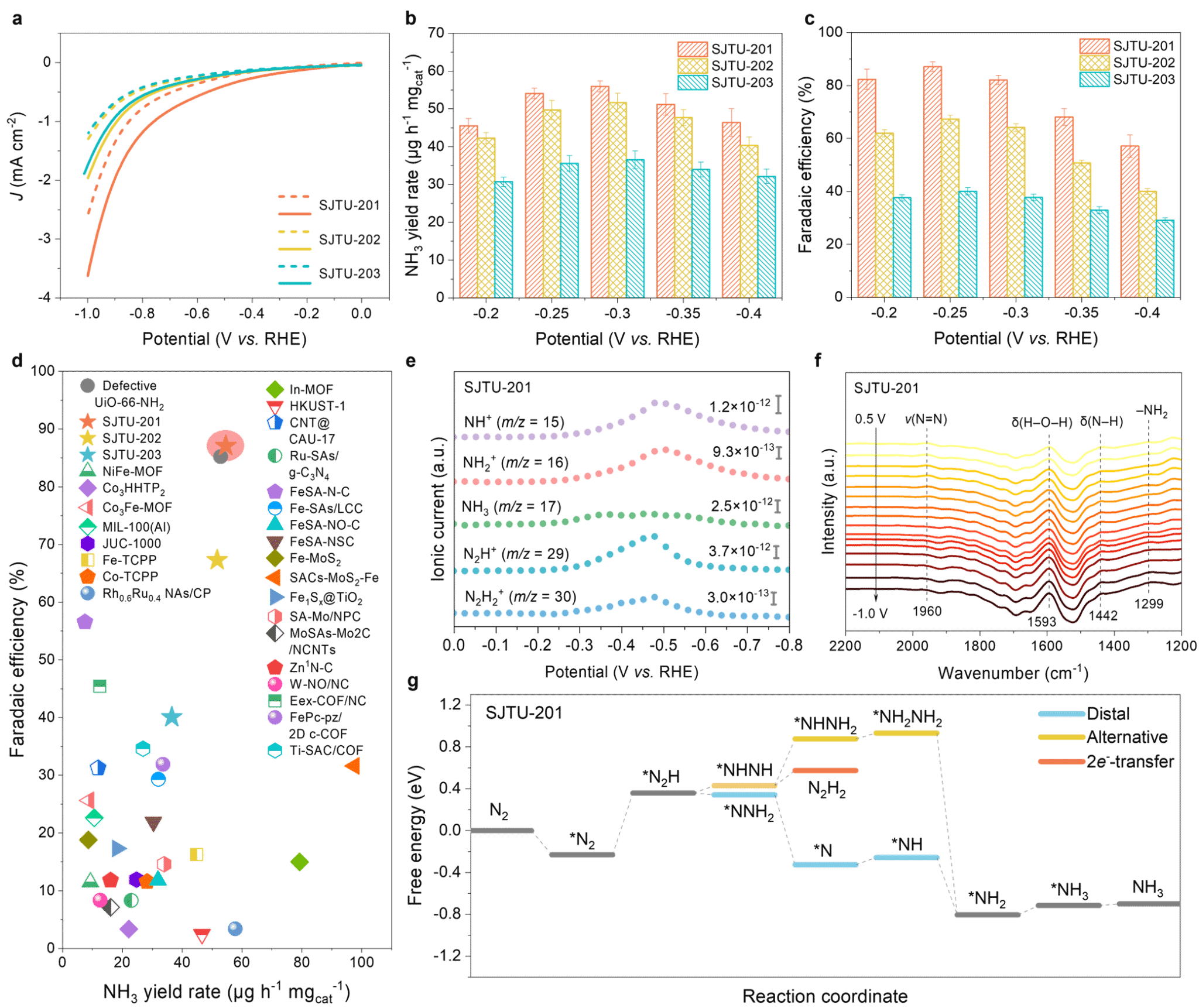
Band structure predictions from density functional theory calculations indicated that the synthesized 2D MOFs possess extremely narrow direct bandgaps (0.10–0.28 eV). Terahertz spectroscopy tests revealed that these 2D MOFs have ultra-high intrinsic carrier mobility, with values reaching up to 560 ± 46 cm² V⁻¹ s⁻¹ (Figure 5). MOFs with well-defined Rh–C₄ active sites demonstrated outstanding catalytic performance in electrocatalytic nitrogen reduction reactions, achieving a Faradaic efficiency of up to 87.1 ± 1.8% for the conversion of nitrogen to ammonia. Online differential electrochemical mass spectrometry and in situ attenuated total reflectance surface-enhanced infrared absorption spectroscopy revealed 2-electron (2e⁻) transfer and distal reaction pathways occurring on the material surface (Figure 6). This work not only expands the structural map of 2D network chemistry with ultra-high carrier mobility, but also marks a milestone in the development of metal–Cx active sites in the field of electrocatalysis.
This work has been published online in Advanced Materials (Adv. Mater. 2025, 2502192) under the title "2D Rhodium–Isocyanide Frameworks".
The co-first authors are Shenhe Huang (doctoral student, School of Chemistry and Chemical Engineering, SJTU), Pu Yan (doctoral student, School of Physical Science and Technology, ShanghaiTech University), and Zhiya Han (School of Materials, Shanghai Dianji University).
The project was supported by the National Natural Science Foundation of China, the Shanghai Science and Technology Commission, and the China Postdoctoral Science Foundation.
Original link:
https://advanced.onlinelibrary.wiley.com/doi/epdf/10.1002/adma.202502192







 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn