搜索


Recently, the team of Professor Xudong Qu from the Zhangjiang Institute for Advanced Study and the School of Life Sciences and Biotechnology at Shanghai Jiao Tong University, in collaboration with Mehdi Mobli's team from the University of Queensland, and the teams of Yilei Zhao and Xudong Kong from the same school, published a research paper in Nature Chemistry titled:“An enzymatic dual-oxa Diels-Alder reaction constructs the oxygen-bridged tricyclic acetal unit of (-)-anthrabenzoxocinone”.For the first time, they revealed the catalytic mechanism of a novel dual-oxa Diels-Alder (HDA) enzyme. This enzyme is responsible for forming the chiral oxygen-bridged tricyclic acetal unit in the type II polyketide natural product (-)-anthrabenzoxocinone (ABX).Postdoctoral researcher Xiaoli Yan from SJTU and Dr. Xinying Jia from the University of Queensland are co-first authors.Professors Xudong Qu, Mehdi Mobli, and Yilei Zhao are co-corresponding authors.Tenured associate professor Xudong Kong, and students Shunjia Ji and Mengjie Zhang are co-authors.
Hetero-Diels-Alder (HDA) reactions are among the most efficient ways to construct six-membered heterocycles, with wide applications in medicinal chemistry, materials science, and natural product synthesis.Among them, oxa-HDA reactions (i.e., when the diene or dienophile contains an oxygen atom) are the most common, enabling efficient construction of oxygen-containing heterocyclic skeletons.Although multiple enzymes that catalyze single oxa-HDA reactions (such as LepI, EupfF, Tsn11, etc.) have been discovered in nature, dual-oxa Diels-Alder reactions (involving two oxygen atoms in a [4+2] cycloaddition) have never been reported before. Their catalytic mechanism and enzymological basis remain entirely unknown.
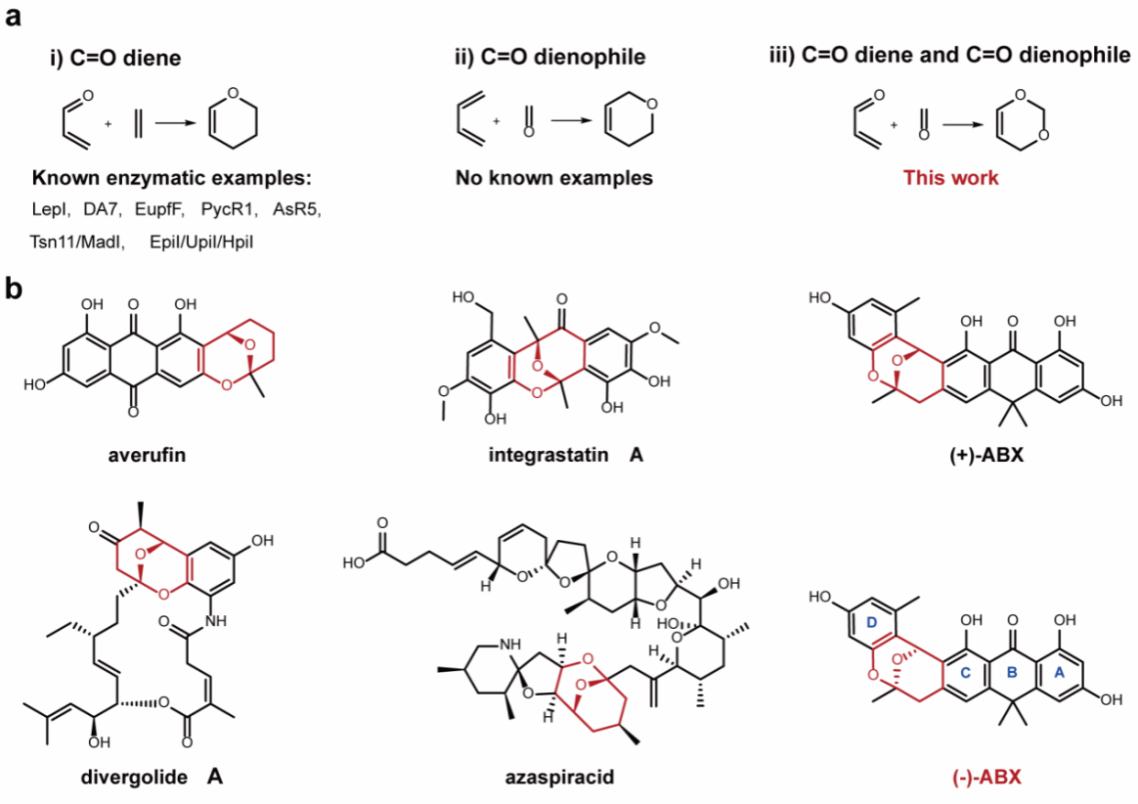
Prof. Xudong Qu’s group has long been devoted to research on the biosynthesis of molecular skeletons. Their previous work has elucidated the formation mechanism of the tetracyclic aromatic core in (-)-ABX (Proc Natl Acad Sci USA 2024, 121, e2321722121).However, the biosynthetic mechanism of the tricyclic oxygen-bridged acetal unit—a key structural element—has remained unresolved.It is worth noting that this type of tricyclic oxygen-bridged acetal structure is widely present in a variety of natural products with significant biological activity (Figure 1b). Its unique rigid conformation critically impacts the pharmacological activity of the molecule.Therefore, decoding its biosynthetic mechanism is of great significance for natural product chemistry and innovative drug development.
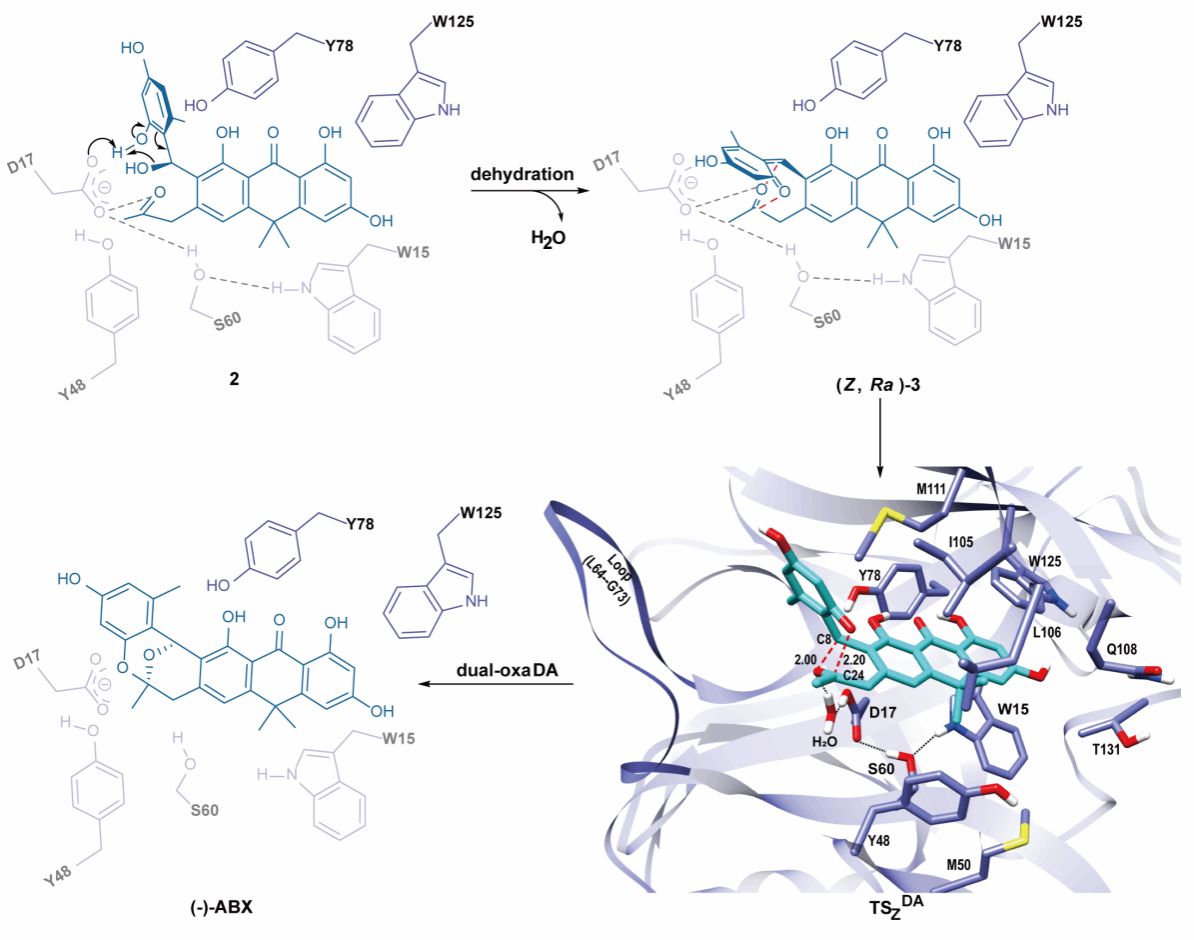
In this project, the joint research team conducted systematic in vivo and in vitro experiments and discovered for the first time that the VOC superfamily protein Abx(-)F is a DA enzyme with dual catalytic functions:First, it catalyzes a dehydration reaction to convert compound 2 into an o-quinone methide (o-QM) intermediate (Z, Ra)-3 with specific axial chirality;Then, it stereoselectively catalyzes the intramolecular dual-oxa Diels-Alder reaction of (Z, Ra)-3, precisely constructing the chiral tricyclic oxygen-bridged acetal structure.The research team further applied X-ray crystallography and nuclear magnetic resonance (successfully resolving the structure of Abx(-)F and its complex with the substrate), as well as computational chemistry, to unveil a new catalytic mechanism called “dehydration-coordination synergistic HDA” (Figure 2), providing a paradigm shift for understanding the biosynthesis of such complex structures.
The scientific significance of this research manifests in multiple dimensions:
(1)New enzymological discovery: The identification of the first dual-oxa HDA enzyme not only fills a gap in the study of multi-heteroatom Diels-Alderases but also suggests the possible existence of a novel family of multi-heteroatom HDA enzymes in nature, greatly expanding our understanding of enzymatic HDA reactions.
(2)Breakthrough in natural product synthesis: This study provides the first enzymatic template for understanding the widely existing oxygen-bridged acetal structures, solving a longstanding critical scientific question in the field.
(3)New understanding in polyketide chemistry: The oxygen-bridged acetal unit in (-)-ABX represents the ninth scaffold type discovered among nearly 10,000 type II polyketide compounds. It is also the second chiral scaffold reported by the research group, following their recent identification of the first chiral scaffold (J Am Chem Soc 2025, 147, 5596-5601), laying a crucial foundation for the rational design of complex aromatic polyketides.
This research not only deepens the understanding of the chemical diversity of HDA reactions but also provides innovative tools for the targeted biosynthesis and enzyme engineering of natural products.The related technology is expected to be applied to the green manufacturing of high-value oxygen-containing heterocyclic compounds.
This work was supported by the National Key R&D Program, the National Natural Science Foundation of China, the Shanghai Academic/Technology Leader Program, and other projects.
Paper link: https://doi.org/10.1038/s41557-025-01804-0






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn