搜索


Research Background:
Lithium metal has long been regarded as an ideal anode material for high-energy-density batteries. However, its application is limited due to the tendency of lithium metal to form dendrites at practical current densities. In solid-state lithium batteries, primary failures typically stem from mechanical issues, where lithium dendrites can penetrate and/or disrupt the solid electrolyte. Despite decades of efforts to minimize dendrite formation, complex mechanisms and interdependent factors continue to hinder the large-scale commercialization of rechargeable lithium anodes.
Research Overview:
Recently, Professor Luo Jiayan from Shanghai Jiao Tong University, in collaboration with Associate Researcher Wang Aoxuan and Dr. Zhang Xinyue from Tianjin University, alongside CATL Chief Scientist Wu Kai and Argonne National Laboratory Researcher Liu Tongchao, pioneered the use of phase-field modeling to simulate and validate that smoother morphologies form more readily on Li surfaces with lower self-diffusion barriers. They introduced a strategy to directly convert commercial polycrystalline lithium (poly-Li) into various single-crystal lithium metal anodes with uniform crystal planes via recrystallization techniques. Simultaneously, by elucidating the diffusion kinetics and mechanical properties of different lithium crystal planes, it was discovered that single-crystal Li(110) exhibits the lowest diffusion barrier (0.02 eV)—an order of magnitude lower than the 0.2 eV barrier of polycrystalline lithium—with its Young's modulus reduced by 71% from 9.42 GPa to 2.71 GPa. Further results demonstrate that employing single-crystal Li(110) in solid-state batteries elevates the critical current density by an order of magnitude, a finding consistently validated across diverse inorganic solid electrolytes including oxides, halides, and sulfides. Moreover, researchers demonstrated that pairing single-crystal Li(110) with high-nickel cathodes extends the cycling stability of lithium metal batteries by fivefold. This strategy of controlling crystal planes effectively addresses a key challenge in achieving high-energy-density batteries.
Key research findings:
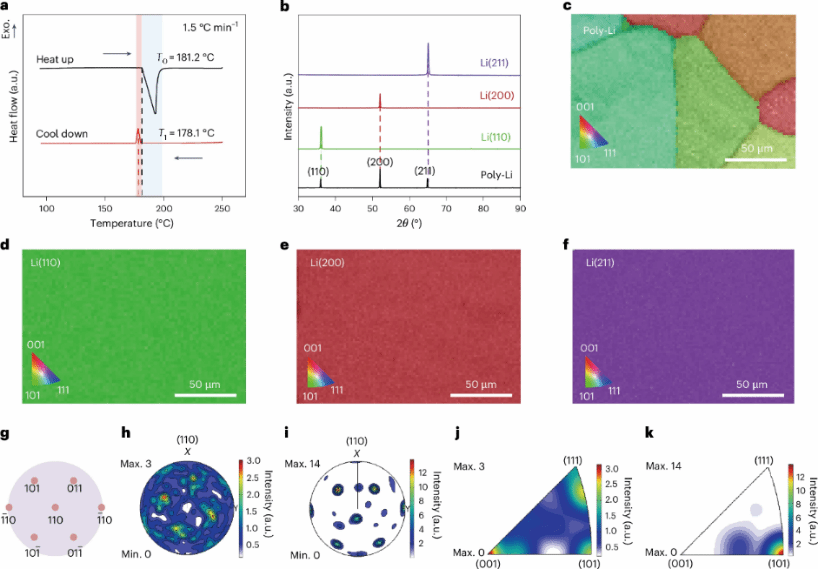
Figure 1 Single-crystal lithium with different crystal planes. a, DSC measurements of lithium metal at heating/cooling rates of 1.5 °C min⁻¹. The blue shaded region represents the endothermic process during lithium metal melting, while the red shaded region indicates the exothermic phase. b, XRD patterns of polycrystalline lithium and single-crystal lithium with individual Li(110), (200), and (211) crystal planes. c–f, EBSD IPF maps of polycrystalline lithium (c), Li(110) (d), Li(200) (e), and Li(211) (f). g, Standard XRD projection pattern of the Li(110) plane. h, i, XRD PF of polycrystalline lithium (h) and Li(110) (i). j, k, XRD IPF of polycrystalline lithium (j) and Li(110) (k).
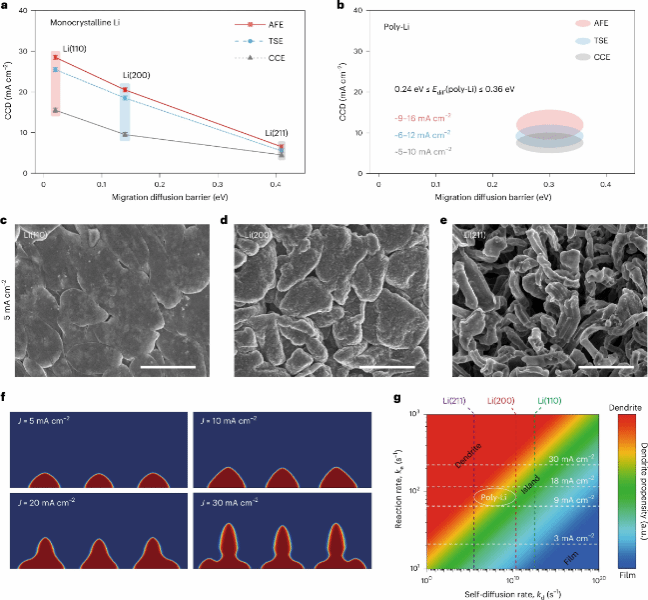
Figure 2 Kinetic characteristics of single-crystal lithium on different crystal planes. a, b Critical current density (CCD) of single-crystal lithium metal anodes (a) and polycrystalline lithium metal anodes (b) in three different electrolytes. Each data point represents the average of the maximum and minimum current densities within the CCD step interval. The total span of each error bar represents the step interval of current density. c–e, SEM images of Li deposition morphology on Li(110) (c), Li(200) (d), and Li(211) (e) at a current density of 5 mA cm⁻² with a fixed deposition capacity of 10 mAh cm⁻² in AFE. Scale bar, 3 μm. f, Phase-field model simulation of Li deposition morphology on Li(110) at applied current density (J) (t = 7,200 s). g, Predicted phase diagram of deposition morphology in AFE based on self-diffusion rate and reaction rate.
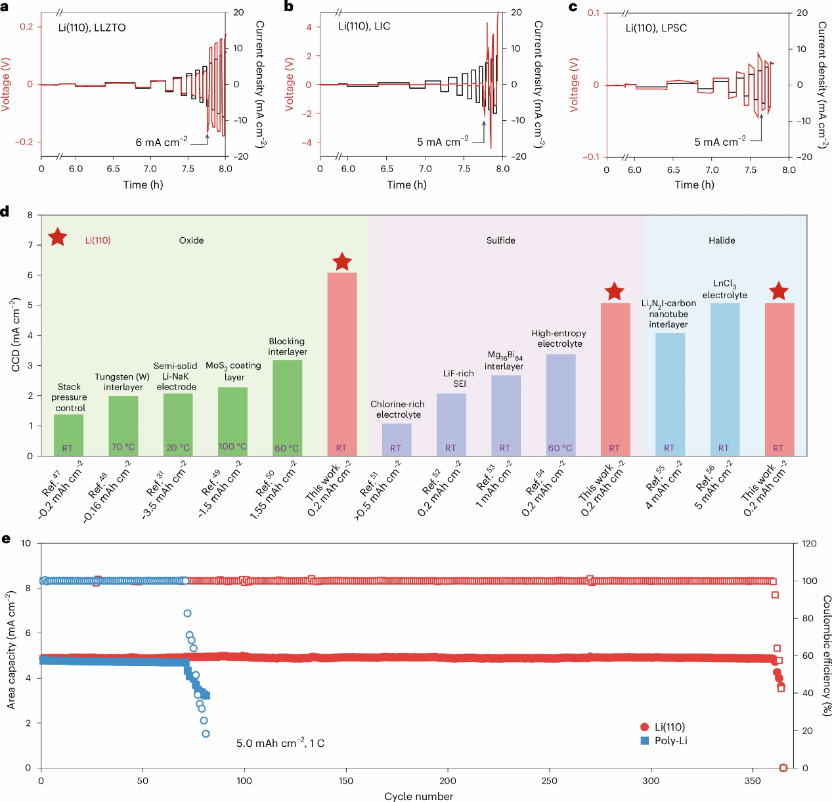
Figure 3 Electrochemical performance of single-crystal Li(110) metal anodes with solid-state electrolytes. a–c, CCD of single-crystal Li(110) symmetric cells based on LLZTO (a), LIC (b), and LPSC (c) solid-state electrolytes. Pressure applied: 5 MPa. d, Comparison of CCD for the three solid-state electrolyte types with Li(110) anode strategies and other reported modification methods. RT denotes room temperature. e, Cycling performance of solid-state Li/In || LPSC || NCM811 batteries at 1 C. LiNi₀.₈Co₀.₁Mn₀.₁O₂ (NCM811) cathode loaded at approximately 35.67 mg cm⁻².
Conclusions and Outlook:
Researchers report that recrystallized single-crystal Li(110) can withstand critical current densities (CCD) in solid-state batteries that are one order of magnitude higher than commercial lithium anodes. Its ultra-low self-diffusion barrier and low Young's modulus mitigate stress accumulation at the lithium metal anode/solid electrolyte interface. Lithium metal batteries utilizing single-crystal Li(110) demonstrate long-term cycling performance in both liquid and solid electrolytes, establishing a new paradigm for high-energy-density rechargeable batteries employing metallic anodes through crystallographic engineering.
The paper was published in Nature Synthesis. First author Hao Chen is a 2021 doctoral student at the School of Materials Science and Engineering, Shanghai Jiao Tong University. Corresponding author Professor Jia-Yan Luo is from the School of Materials Science and Engineering, Shanghai Jiao Tong University, and the Center for Future Materials Innovation at the Zhangjiang Advanced Research Institute. Collaborating institutions include Tianjin University, CATL, and Argonne National Laboratory. This work was funded by the Key Research and Development Program of the Ministry of Science and Technology.
Article link: https://doi.org/10.1038/s44160-024-00712-4
Author: Luo Jia-Yan Team
Contributing Unit: Center for Future Materials Innovation






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn