搜索


Recently, the international authoritative journal The Proceedings of the National Academy of Sciences (PNAS) published online the collaborative research findings of the Ultrafast Science Research Center at Zhangjiang Advanced Research Institute, Shanghai Jiao Tong University, titled “Elucidation of a distinct photoreduction pathway in class II Arabidopsis thaliana photolyase.” Corresponding authors are Professors Ding Bei and Zhong Dongping from the Ultrafast Science Center. First authors are Dr. Zhou Zhongneng, Dr. Chen Zijin, and Dr. Jiang Xiuwen from the Ultrafast Science Center.
Within living organisms, DNA photolyase (PL) and cryptochrome (Cry) play pivotal roles in DNA repair and light sensing, respectively. Both possess a tryptophan (Trp) triplet or a fourth tyrosine (Tyr) to form photoreduction networks (Figure 1), though their quantum yield for DNA repair is lower than that of Class I photolyase. To deepen understanding of the photoreductive mechanism in Class II photolyases, the research team conducted a pioneering study. Utilizing mutation design and femtosecond spectroscopy techniques, they successfully revealed the unique photoreductive mechanism of Arabidopsis Class II photolyase (AtPL). This discovery not only provides new insights into the functional diversity of the photolyase family but also opens new avenues for future research in related fields.
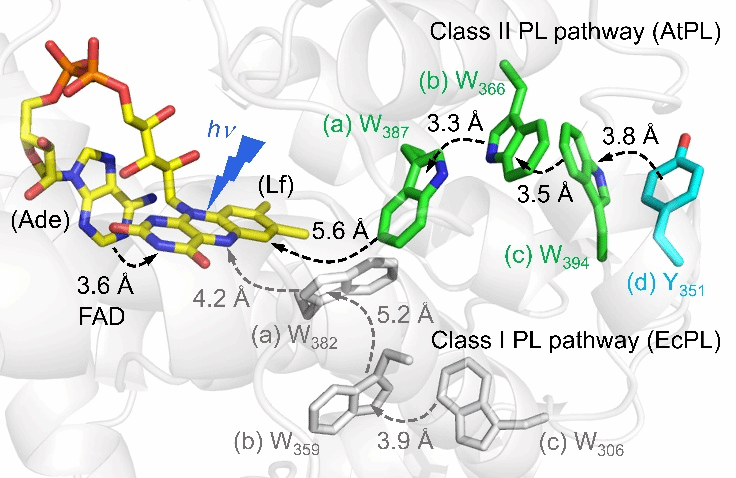
Figure 1. Different photoreduction pathways in Class I and Class II photolyases. Dashed arrows indicate the direction of electron flow. Class I photolyase pathway: The classical Trp triplet pathway in Escherichia coli photolyase (EcPL, PDB code 1DNP) is shown in gray. Class II Trp pathway: The electron-transferring Trp triplet and fourth Tyr in AtPL are shown in green and blue, respectively, with their electron-transfer distances marked. The AtPL structure was predicted using Alphafold3.
By designing the W387F mutant to block the electron transfer chain in AtPL, the research team discovered that the intramolecular electron transfer (ET) between the flavin (Lf) and adenine (Ade) moieties of the flavin adenine dinucleotide (FAD) cofactor in AtPL exhibits unique reverse kinetics. The forward electron transfer (fET) time constant between them is 172 ps, while the reverse electron transfer (rET) time constant is only 26 ps (Figure 2), starkly contrasting with the normal kinetics observed in Class I EcPL [1]. By estimating the electron transfer reorganization energy using Marcus electron transfer theory, we found that the reorganization energies differ between the two classes of photolyases. This may be attributed to the presence of more water molecules in the FAD cavity of Class II photolyases due to structural reasons.
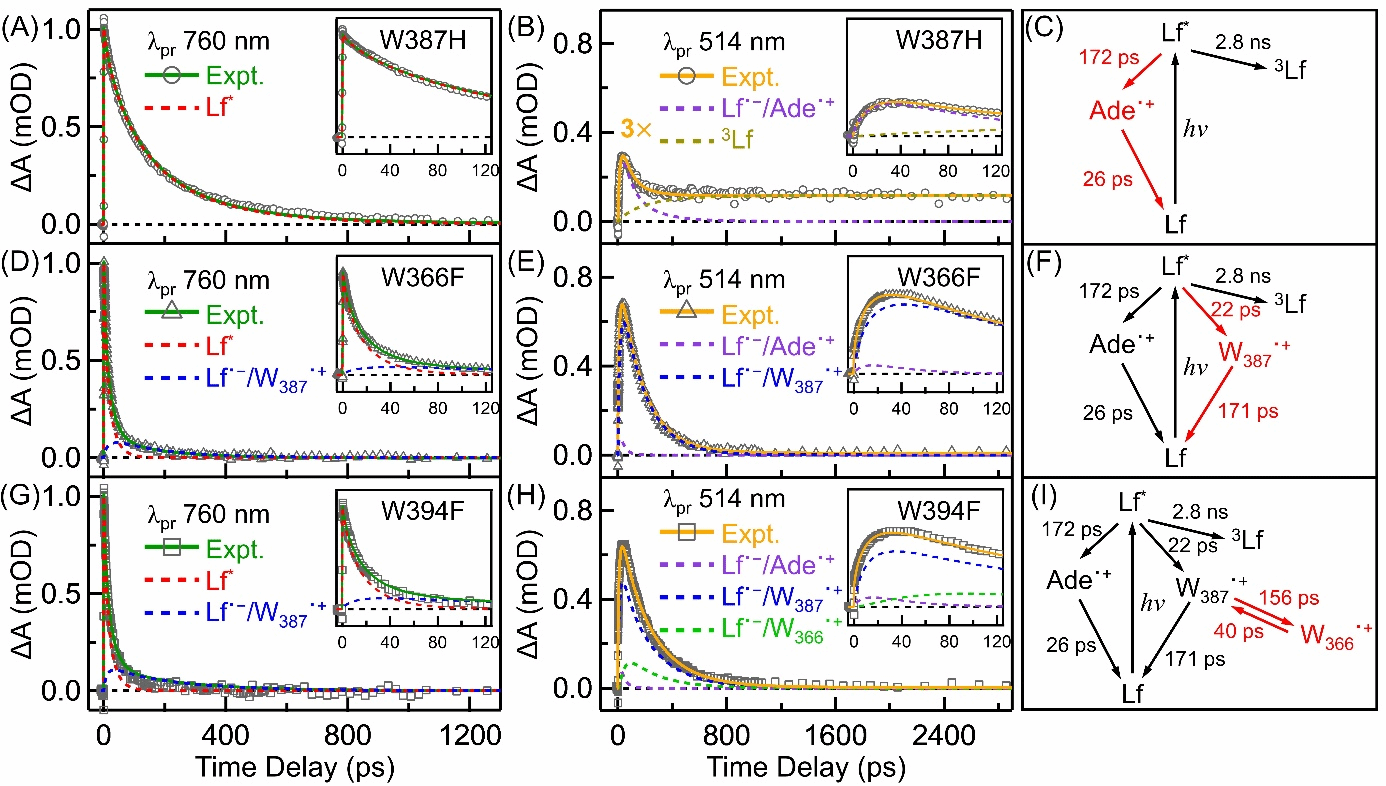
Figure 2. Transient absorption kinetics at specific wavelengths were analyzed. The figure shows the transient kinetic decomposition of W387H, W366F, and W394F at 760 nm (A, D, G) and 514 nm (B, E, H). The signal intensity of the W387H mutant (B) at 514 nm was amplified by a factor of 3. Deconstructed kinetic curves include Lf*, Lf˙⁻/Ade˙+, Lf˙⁻/W387˙+, Lf˙⁻/W366˙+, and 3Lf. The inset depicts kinetic curves plotted up to 120 ps. Kinetic models and fitted parameters for W387H (C), W366F (F), and W394F (I) are also shown on the right, with fitted parameters in red and parameters fixed in the analysis in black.
The research team investigated the electron transfer rate from Lf to the nearest tryptophan (Wa) in the proposed atPL, conducting a series of experiments using the W366F mutant. Data analysis identified a forward electron transfer (fET) time of 22 ps and a reverse electron transfer (rET) time of 171 ps between W387 and Lf in W366F (Figure 2). Estimated using Marcus electron transfer theory, this fET exhibits a ΔG0 of approximately -0.58 eV and a recombination energy of 1.10 eV (fET). Experiments on the W394F mutant also revealed that the electron transition from Wa to Wb is energetically unfavorable. Utilizing the fET (156 ps) and rET (40 ps) lifetimes between them, the ΔG0 for this fET was estimated to be approximately 0.04 eV. This contrasts with the energetically favorable electron transitions observed in Class I EcPL [1], indicating a unique electron transfer mechanism in AtPL. This energetically uphill ET step explains why the photoreduction quantum yield of Class II AtPL (approximately 34%) is lower than that of Class I EcPL (approximately 45%).
By engineering the Y351F mutant, the research team also captured the rapid deprotonation of the distal Trp residue Wc in Arabidopsis Class II photolyase (Figure 3). Wc rapidly loses a proton within 3 ns during photoreduction, generating the neutral radical Wc˙. This phenomenon also echoes the rapid deprotonation of Wc in Class II MmPL [2]. This rapid deprotonation not only highlights the uniqueness of the photoreduction mechanism in Class II photolyases but may also be closely related to their protein motion. Future studies will further explore its biological significance and role in photolyase evolution. This study provides an in-depth investigation of the photoreduction mechanism in Class II AtPL, which will aid future design of artificial photosystems and photoreceptor research. Furthermore, it raises important scientific questions about the unique photoreduction network of Class II photosystems and why their protein frameworks have evolved in this manner.
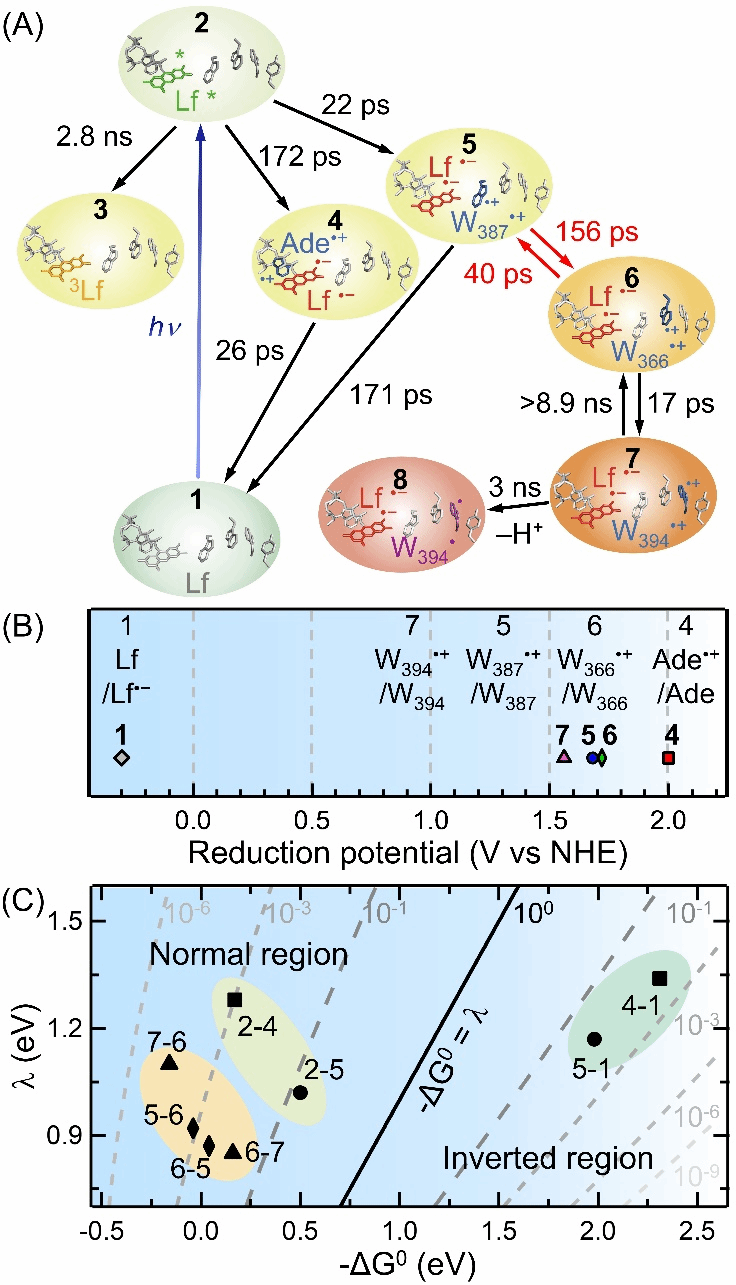
Figure 3. The figure summarizes the elementary reaction steps formed during AtPL photoreduction, along with their redox potentials and relationships to driving forces and recombination energies. (A) The photoreduction network of AtPL wild-type (8 electron transfer steps and 1 proton transfer step, with fitted time constants). (B) Reduction potentials of intermediate species including Lf/Lf⁻, Ade⁺/Ade, W387⁺/W387, W366⁺/W366, and W394⁺/W394. (C) Two-dimensional plot of electron transfer rates versus free energy (ΔG₀) and recombination energy (λ) for all 8 electron transfer steps. To compare with reported EcPL results [1], a distance-dependent parameter β = 1.4 Å⁻¹ was applied to all 8 electron transfer steps. Among these, the two charge-transfer steps associated with Lf (5-1 and 4-1) occurred in the Marcus inversion region (-ΔG₀ > λ), while the remaining steps occurred in the Marcus normal region (-ΔG₀ < λ).
This work was supported by the Ministry of Science and Technology (2020YFA0509700) and the National Natural Science Foundation of China (22373067, 12004246, 22433003).
Paper link: https://www.pnas.org/doi/abs/10.1073/pnas.2416284121
References:
1. Z. Liu, et al., Determining complete electron flow in the cofactor photoreduction of oxidized photolyase. Proc. Natl. Acad. Sci. U. S. A. 110, 12966–12971 (2013).
2. P. Müller, E. Ignatz, S. Kiontke, K. Brettel, L.-O. Essen, Sub-nanosecond tryptophan radical deprotonation mediated by a protein-bound water cluster in class II DNA photolyases. Chem. Sci. 9, 1200–1212 (2018).
Author: Zhong Dongping Team
Contributing Unit: Center for Ultrafast Science






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn