搜索


Two-dimensional metal-organic frameworks (MOFs) hold great promise in energy devices, optoelectronics, and biological applications due to their chemical tunability, structural anisotropy, and exceptional charge transport properties. These properties stem from two key aspects: the in-plane conjugated network formed by orbital hybridization between metals and ligands, and the out-of-plane van der Waals interactions. Despite their significant potential for photoactivity and catalytic activity, two-dimensional MOFs based on metal-carbon coordination remain rarely reported. This scarcity primarily attributes to their poor chemical stability and complex, demanding synthesis conditions.
The metal-carbon coordination environment has long been a crucial building block for catalyst development in organic synthesis. Metal-organic complexes containing metal-carbon sites play pivotal roles in organic catalysis and drug synthesis. However, such molecular catalysts exhibit poor chemical stability. Two-dimensional MOFs, with their porous and stable lattices, offer ideal carriers for metal-carbon active sites. Although researchers have attempted to introduce metal-carbon sites into 2D MOFs via post-synthesis modification, these methods suffer from low metal loading, poor stability, and difficulties in structural characterization. The direct synthesis of metal-carbon coordination-based 2D MOFs has long been challenging, let alone systematic studies on their electrical properties and catalytic activity. The absence of universal synthetic strategies and suboptimal crystallinity have hindered both structure-property relationship studies and application development for such 2D MOFs.
Recently, Professor Xiaodong Zhuang's team at the School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, synthesized a series of rhodium-isocyanate (Rh-iCN)-coordinated 2D MOFs (Figure 1) via a simple solution-based synthesis under an inert atmosphere. This represents another novel metal-carbon coordination bond-based two-dimensional coordination polymer system following the Ag-iCN coordination group-based two-dimensional MOFs developed in December 2024 (Angew. Chem. Int. Ed. 2025, 64, e202417658; doi.org/10.1002/anie.202417658).
These 2D MOFs were synthesized via the coordination reaction between aryl diisocyanate ligands and chlorinated (1,5-cyclooctadiene)rhodium(I) dimers. Rh-C4 coordination sites centered on Rh(I) were directly incorporated into the 2D MOF lattice, yielding air-stable structures. Powder X-ray diffraction confirmed the synthesized 2D MOFs exhibit high crystallinity and a layered AA stacking pattern (Figure 2). Aberration-corrected high-resolution transmission electron microscopy and electron diffraction analysis confirmed the in-plane quasi-square lattice network structure of the 2D MOFs (Figures 3 and 4).
Density functional theory calculations predicted an extremely narrow direct bandgap (0.10–0.28 eV) for the synthesized 2D MOFs. Terahertz spectroscopy revealed exceptionally high intrinsic carrier mobility in these 2D MOFs, reaching up to 560 ± 46 cm² V⁻¹ s⁻¹ (Figure 5). . MOFs with well-defined Rh-C4 active sites exhibit outstanding catalytic performance in the electrocatalytic nitrogen reduction reaction, achieving a nitrogen-to-ammonia Faradaic conversion efficiency as high as 87.1 ± 1.8%. Online differential electrochemical spectroscopy and in situ attenuated total reflection surface-enhanced infrared spectroscopy reveal the occurrence of two-electron (2e-) transfer and distant reaction pathways on the material (Figure 6). This work not only expands the structural landscape of two-dimensional networked chemistry with ultra-high carrier mobility but also establishes a significant milestone in the development of metal-Cx active sites for electrocatalysis.
This research is currently published online in Advanced Materials under the title “2D Rhodium-Isocyanide Frameworks” (Adv. Mater. 2025, 2502192). Huang Senhe, a doctoral candidate at the School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University; Yan Pu, a doctoral candidate at the School of Physical Science and Technology, ShanghaiTech University; and Dr. Han Zhiyia from the School of Materials Science and Engineering, Shanghai Dianji University, are the co-first authors of this paper. This work was supported by the National Natural Science Foundation of China, the Shanghai Municipal Science and Technology Commission, and the China Postdoctoral Science Foundation.
Original link: https://advanced.onlinelibrary.wiley.com/doi/epdf/10.1002/adma.202502192
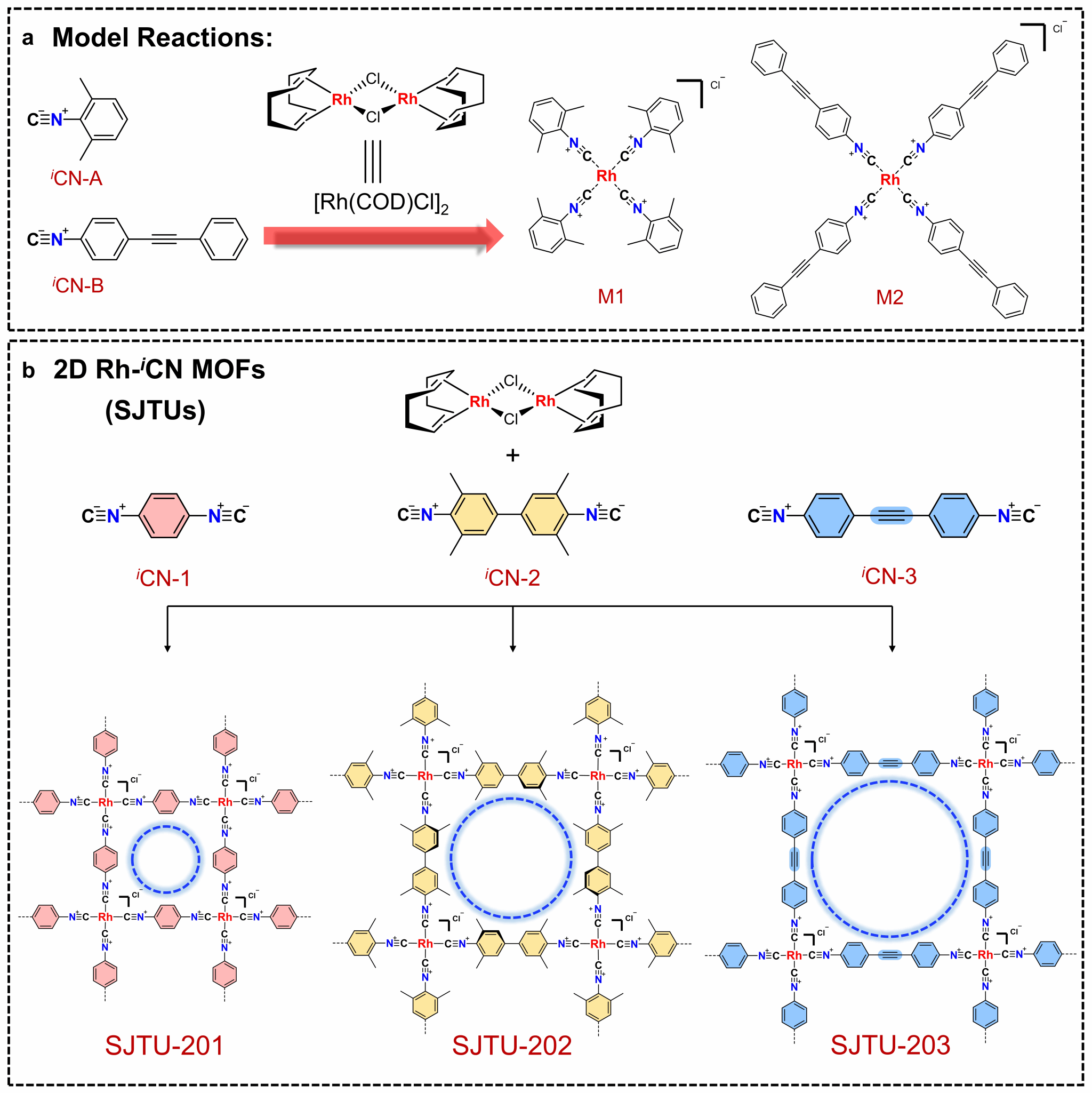
Figure 1. Design Strategy and Synthesis of Two-Dimensional MOFs Based on Rhodium-Isocyanate. (a) Model compound synthesized via coordination between isocyanate and rhodium complexes. (b) Two-dimensional metal-organic frameworks (SJTUs) constructed from different bidentate isocyanate ligands and rhodium complexes.
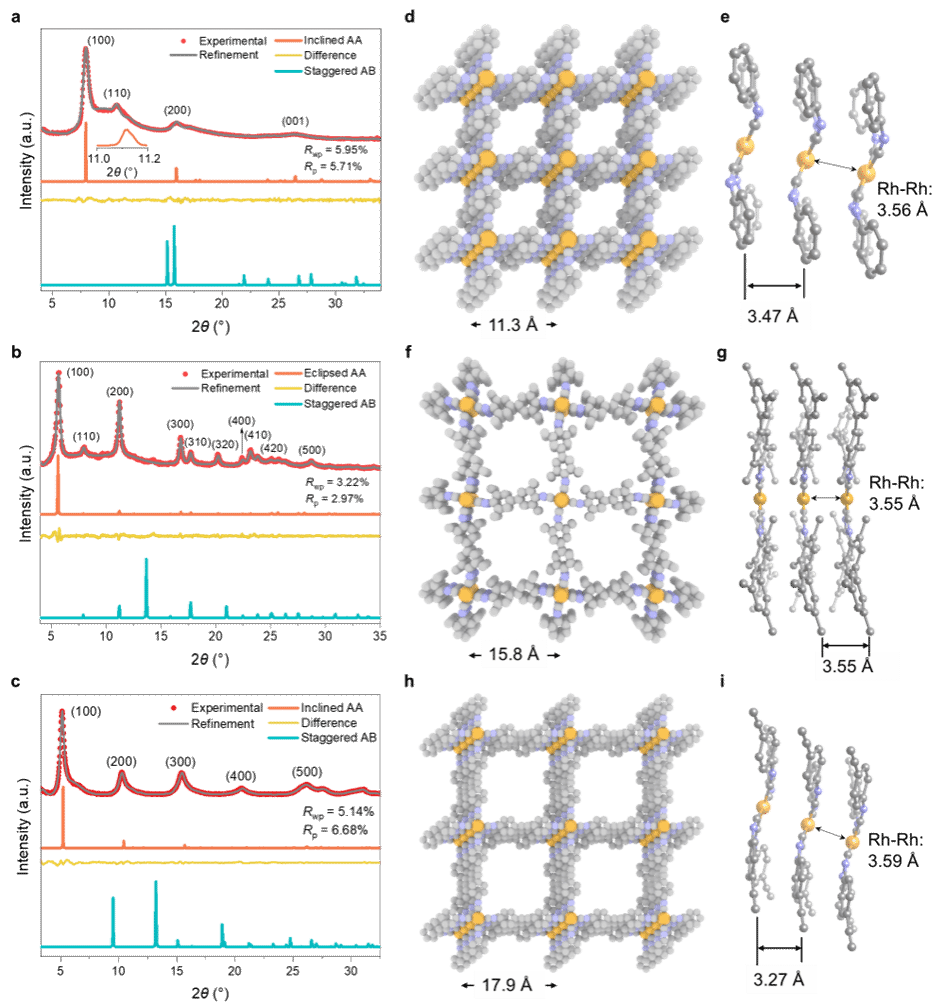
Figure 2. PXRD patterns and simulated structures of SJTUs. (a–c) Experimental (red dots), Pawley-refined (gray lines), computed PXRD patterns and difference maps of SJTUs. SJTU-201 (a); SJTU-202 (b); SJTU-203 (c). Inset: Local magnification of the simulated pattern for SJTU-201. Space-filling models (viewed perpendicular to the ab plane) and corresponding lattice parameters for SJTU-201 (d), SJTU-202 (f), and SJTU-203 (h). Orange, blue, and gray spheres represent Rh, N, and C atoms, respectively. Ball-and-stick models of SJTU-201 (e), SJTU-202 (g), and SJTU-203 (i) (viewed parallel to the ab plane), interlayer spacing, and distance between adjacent Rh atoms in the layers.
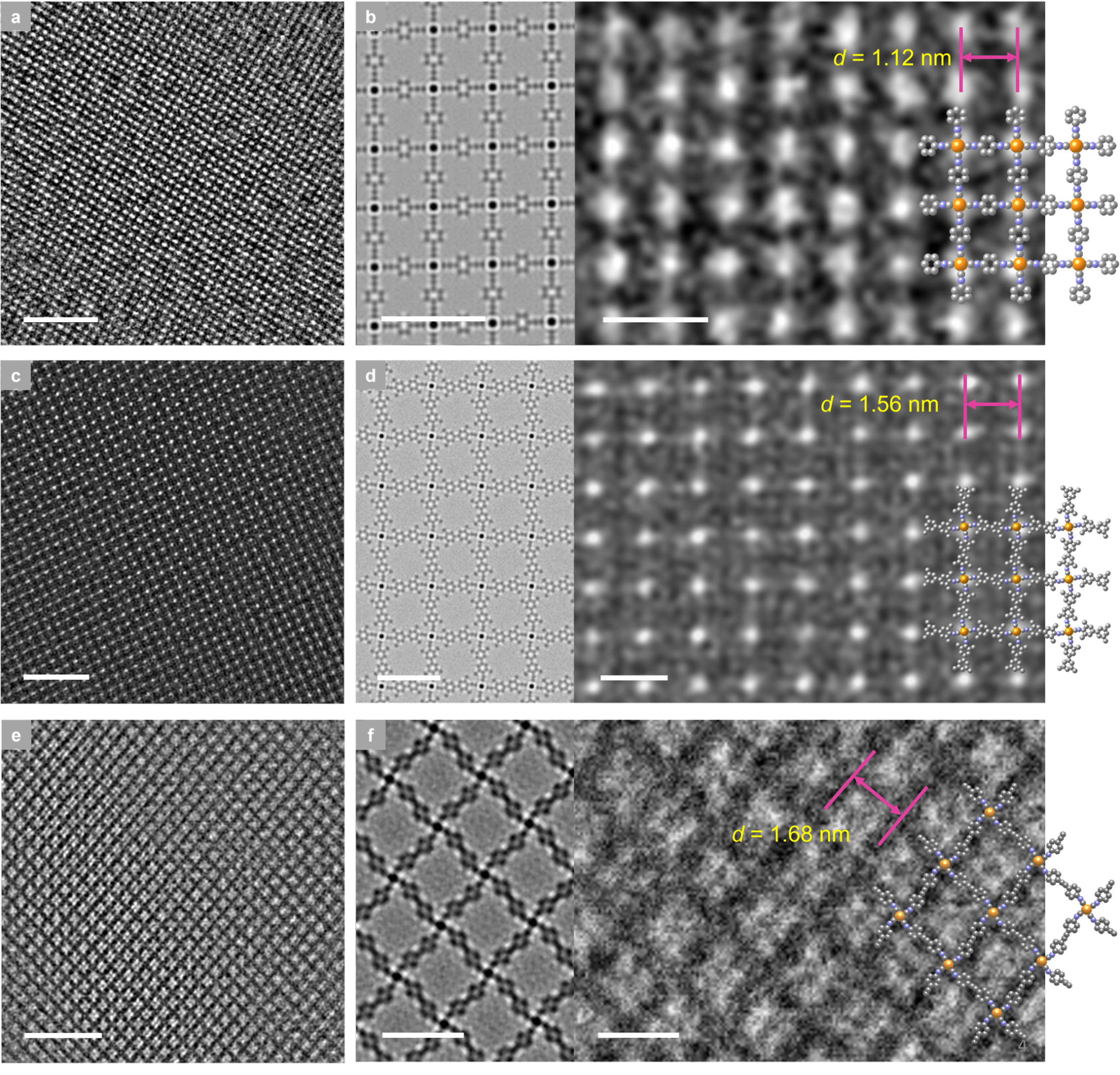
Figure 3. In-plane network structure of SJTUs. AC-HRTEM images of SJTU-201 (a), SJTU-202 (c), and SJTU-203 (e) along the [001] direction reveal square and rhombic pore stacking (scale bar: 10 nm). Enlarged and simulated (left) HRTEM images of SJTU-201 (b), SJTU-202 (d), and SJTU-203 (f), along with interplanar spacings (scale bar: 2 nm). The inset shows the optimized model of SJTUs.
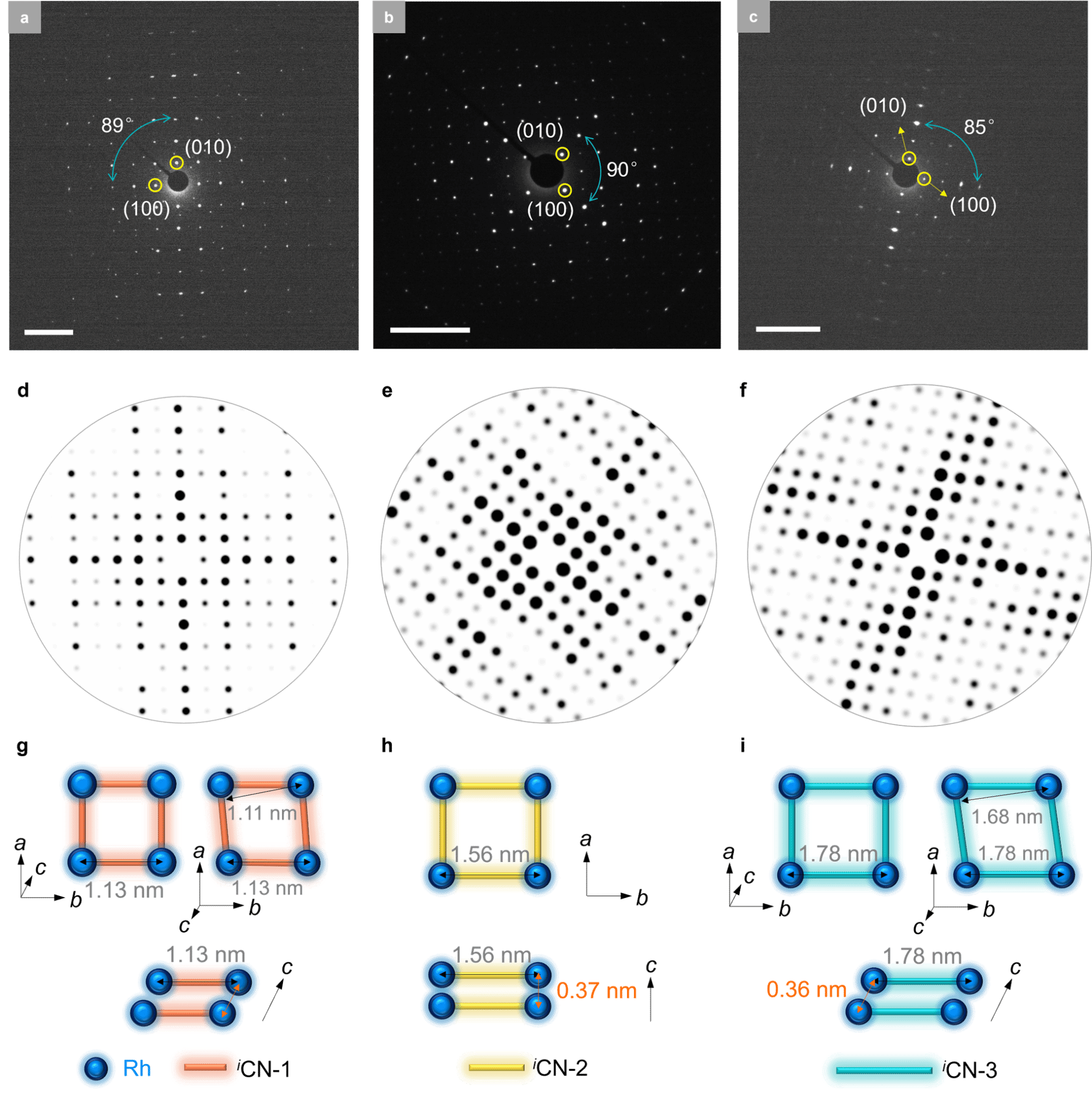
Figure 4. Electron diffraction patterns of SJTUs. (a–c) Experimental electron diffraction patterns of SJTUs observed along the [001] direction. Scale bar: 2 nm⁻¹. SJTU-201 (a); SJTU-202 (b); SJTU-203 (c). (d–f) Simulated electron diffraction patterns of SJTUs observed along the [001] direction. SJTU-201 (d); SJTU-202 (e); SJTU-203 (f). (g–i) Crystal structures of SJTUs derived from electron diffraction pattern analysis. SJTU-201 (g); SJTU-202 (h); SJTU-203 (i).
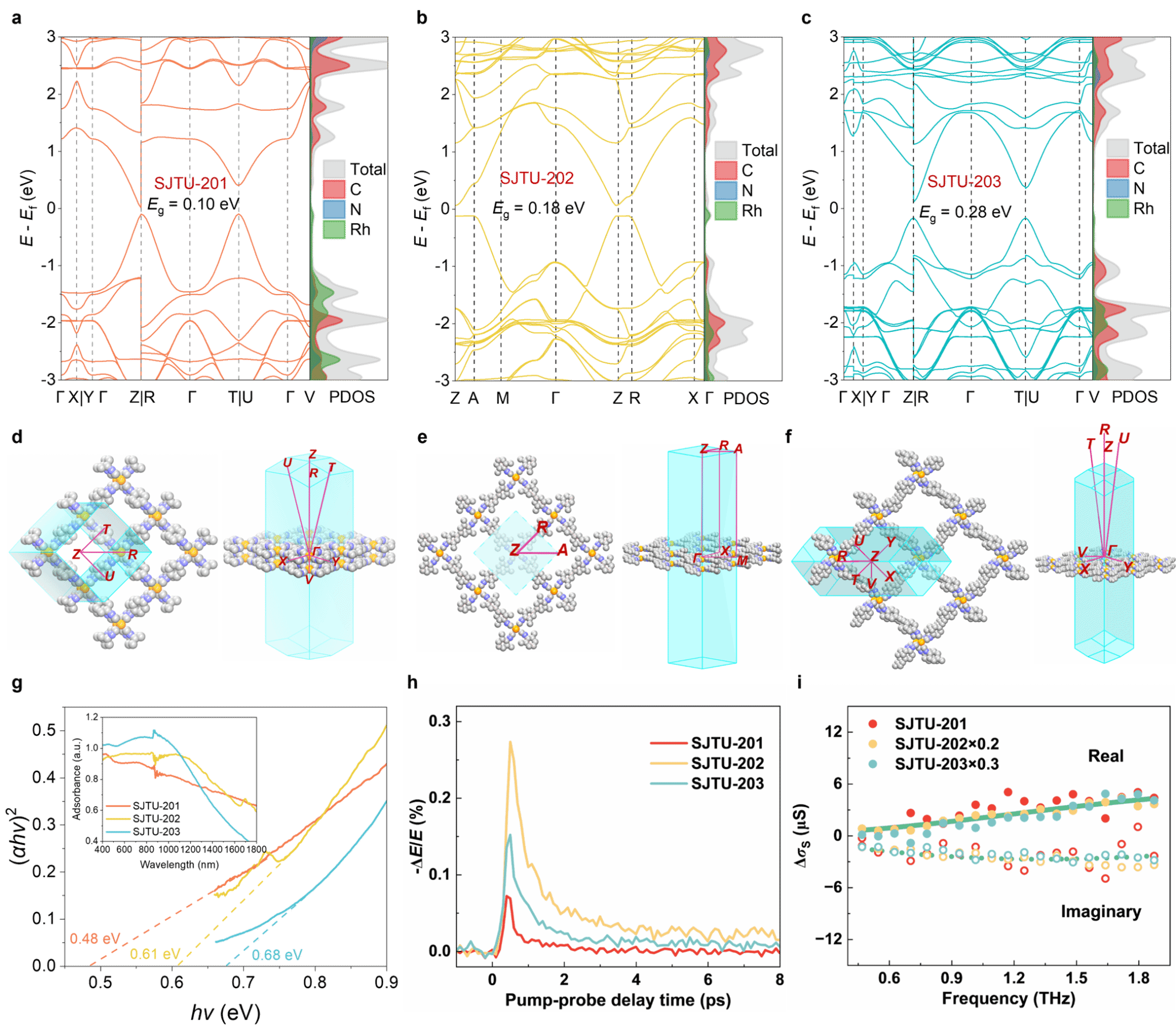
Figure 5. Band structure calculations and charge transport properties of SJTUs. (a–c) Band structures and projected density of states (PDOS) of SJTU-201 (a), SJTU-202 (b), and SJTU-203 (c) calculated using DFT. (d–f) First Brillouin zone and K-paths for SJTU-201 (d), SJTU-202 (e), and SJTU-203 (f). (g) Tauc plot of SJTUs. Inset shows diffuse reflectance UV-vis spectra of SJTUs. (h) Time-resolved terahertz photoconductivity of SJTUs as a function of pump-probe delay time. (i) Frequency-resolved complex terahertz surface photoconductivity of SJTUs measured approximately 1 ps after maximum photoconductivity. The dashed and solid lines correspond to the Drude-Smith model fit for the imaginary and real parts of the complex terahertz surface photoconductivity, respectively.
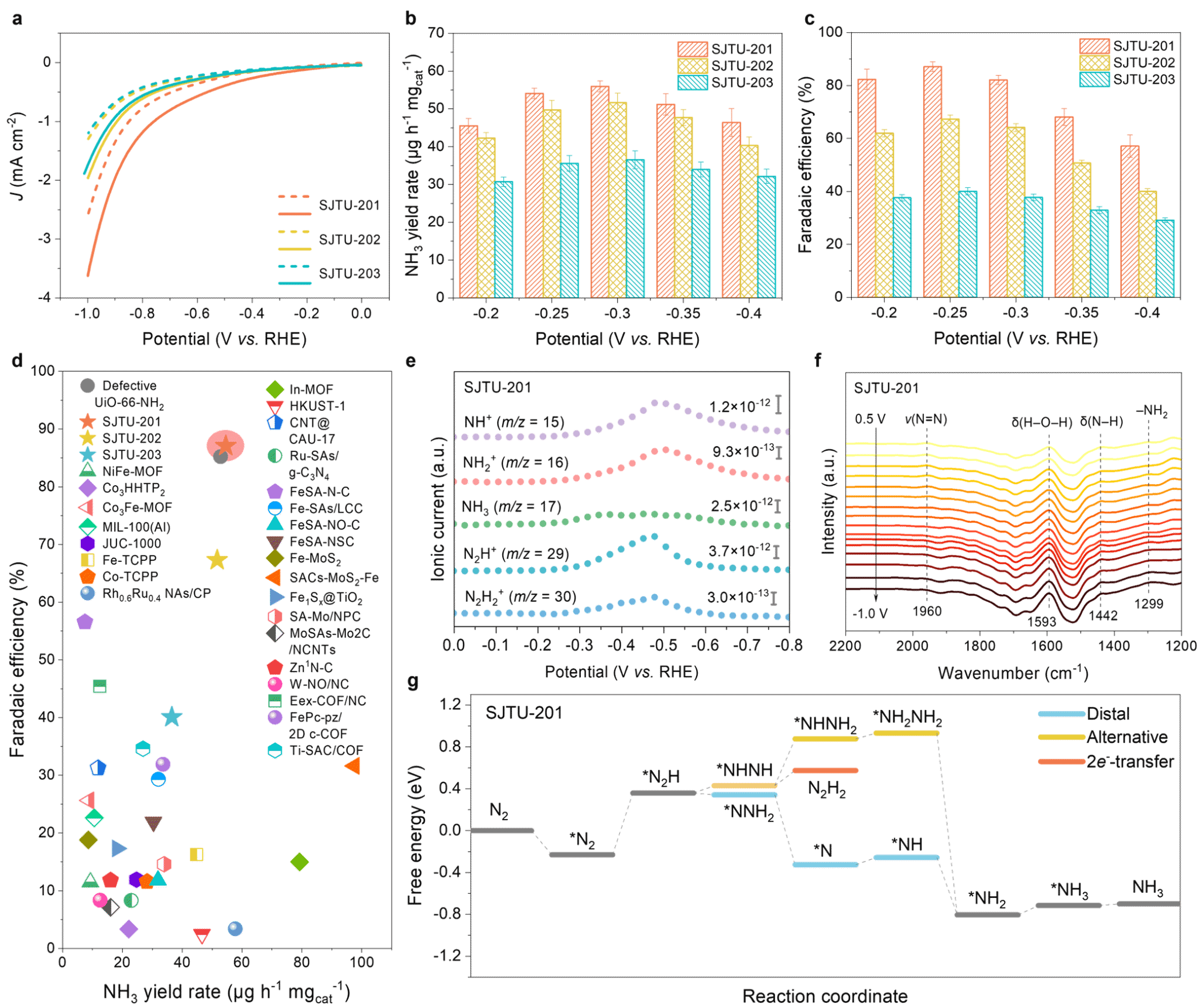
Figure 6. Electrocatalytic NRR performance of SJTUs. (a) LSV curves of SJTUs in 0.1 M K₂SO₄ saturated with Ar and N₂. (b) Faradaic efficiency (FE) of SJTUs at different applied potentials in 0.1 M K₂SO₄ saturated with N₂. (c) NH₃ yield of SJTUs at different applied potentials. (d) Comparison of FE and NH₃ yield of SJTUs with representative reported MOFs, COFs, SACs, and Rh-based catalysts. (e) DEMS spectrum of SJTU-201 versus applied voltage in N₂-saturated 0.1 M K₂SO₄. (f) In situ ATR-SEIRAS spectra of SJTU-201 at different potentials in 0.1 M K₂SO₄. (g) Calculated reaction free energies for the ENRR process (long-range pathway, alternating pathway, and 2e⁻ transfer pathway) of SJTU-201.
Authors: Team of Xiaodong Zhuang
Contributing Unit: Center for Innovation in Synthetic Science






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn