搜索


Recently, Professor Zhuang Xiaodong's team at the Center for Innovation in Synthetic Science, Zhangjiang Advanced Research Institute, Shanghai Jiao Tong University, successfully synthesized a highly crystalline three-dimensional graphitylene framework featuring a ThSi₂ topological structure through meticulously designed heteromonomers. This breakthrough research introduces a novel diyne-linked member to the family of three-dimensional covalent organic frameworks (COFs). Moreover, it successfully reduces the bandgap of graphitylene materials to 1.15 eV—the latest record for covalent framework materials without π-π stacking interactions. This achievement provides a novel design direction for the development of optoelectronic and energy devices using such materials.
Since their first synthesis in 2010, two-dimensional graphitylene (GDYs) have demonstrated immense potential in energy storage, photodetection, and biosensing due to their unique electronic properties and structural diversity. However, conventional synthesis of two-dimensional GDYs has largely been confined to planar stacked structures, while the preparation of crystalline three-dimensional GDY frameworks remains highly challenging. The primary obstacles lie in the lack of suitable monomer design and driving forces for ordered three-dimensional self-assembly. Furthermore, bandgap tuning of GDYs has been a key research focus, yet achieving a balance between narrow bandgaps and high crystallinity has proven difficult, limiting their application in optoelectronic devices.
The research team designed two isomeric monomers with C1 and C2 axis symmetry—tetra-alkyne monomers based on 2,2'-binaphthalene (bNap) and 6,6'-bisazulene (bAz), termed TEbNap and TEbAz. Unlike conventional C3-symmetric monomers, these non-planar monomers formed three-dimensional graphite diyne frameworks on copper foam substrates via Glaser-Hay coupling reactions, named GDY-bNap and GDY-bAz, respectively (Figure 1). Powder X-ray diffraction (PXRD) and high-resolution transmission electron microscopy (HR-TEM) analyses confirmed that both frameworks possess non-interpenetrating ThSi₂ topological structures with excellent crystallinity, exhibiting pore sizes of 1.6 nm and 1.7 nm, respectively (Figure 2). Through diffuse ultraviolet-visible spectroscopy and density functional theory (DFT) calculations, it was discovered that GDY-bAz exhibits an ultra-narrow bandgap of only 1.15 eV due to the large dipole moment of its naphthalene units, significantly lower than the 2.33 eV of GDY-bNap.
The three-dimensional graphitylene framework, with its dual-continuous channels and tunable electronic structure, serves as an ideal carrier for loading single-atom metals. The team electrochemically deposited ruthenium (Ru) single atoms onto GDY-bAz and GDY-bNap, preparing Ru/GDY-bAz and Ru/GDY-bNap catalysts (Figure 3). X-ray absorption fine structure (XAFS) and aberration-corrected high-angle annular-dark field scanning transmission electron microscopy (HAADF-STEM) revealed uniform distribution of Ru atoms in a single-atom state, forming stable Ru-C coordination with the diyne bonds. In electrocatalytic nitrogen reduction reaction (ENRR) testing, Ru/GDY-bAz demonstrated outstanding performance, achieving an ammonia yield of 188.7 ± 1.6 μg h⁻¹ mgcat⁻¹ at a low potential of -0.2 V vs RHE, with a Faradaic efficiency (FE) of 37.4 ± 0.6%, significantly surpassing Ru/GDY-bNap (89.3 ± 1.5 μg h⁻¹ mgcat⁻¹, FE 24.3 ± 0.8%, Figure 4). Online differential electrochemical mass spectrometry (DEMS) and in situ Raman spectroscopy revealed that Ru/GDY-bAz catalyzes nitrogen reduction via an alternative pathway, with the formation of the key intermediate N₂H₂ significantly enhancing its catalytic efficiency (Figure 5). DFT calculations further indicate that GDY-bAz's narrow bandgap and enhanced charge transfer capability lower the reaction energy barrier, enabling it to maintain high selectivity against competing hydrogen evolution reactions (Figure 6). This work not only achieves the rational synthesis of a three-dimensional graphitylene framework but also effectively modulates the bandgap by introducing azide units, providing new insights for developing narrow-bandgap, highly crystalline covalent organic frameworks. Moreover, the outstanding performance of single-ruthenium catalysts based on such frameworks in electrocatalytic nitrogen reduction demonstrates their significant potential for sustainable ammonia synthesis.
The study, titled “Rational Synthesis of Isomeric Graphdiyne Frameworks towards Single-Ruthenium Catalysts and High-Performance Nitrogen Reduction,” has been published in Advanced Materials. Dr. Feng Boyan from Shanghai Jiao Tong University, postdoctoral researcher Zhang Dong from ShanghaiTech University, and Dr. Han Zhiyia from Shanghai Dianji University are the co-first authors. The research was supported by the National Natural Science Foundation of China, the Shanghai Pujiang Program, and the China Postdoctoral Science Foundation. This marks another instance of isomeric narrow-bandgap framework materials following the 2023 work on isomeric two-dimensional covalent organic frameworks (J. Am. Chem. Soc. 2023, 145, 26871-26882).
Original link: https://advanced.onlinelibrary.wiley.com/doi/10.1002/adma.202502980?af=R
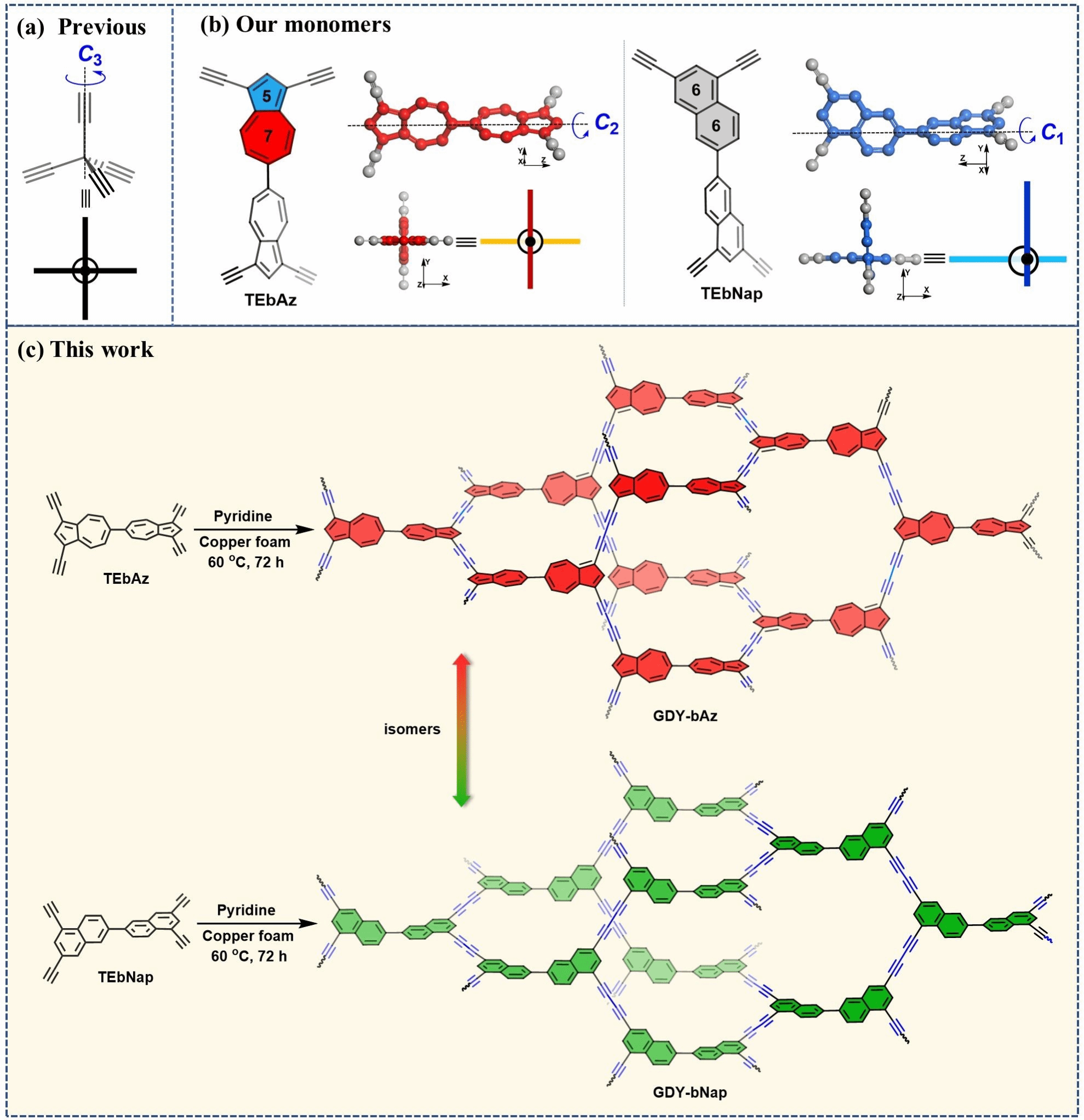
Figure 1. a) Previously reported tetraalkynylmethane monomers exhibiting C3 axial symmetry. b) Isomeric monomers with C1 and C2 axial symmetry synthesized in this work. c) Synthesis of isomeric graphitylene frameworks.
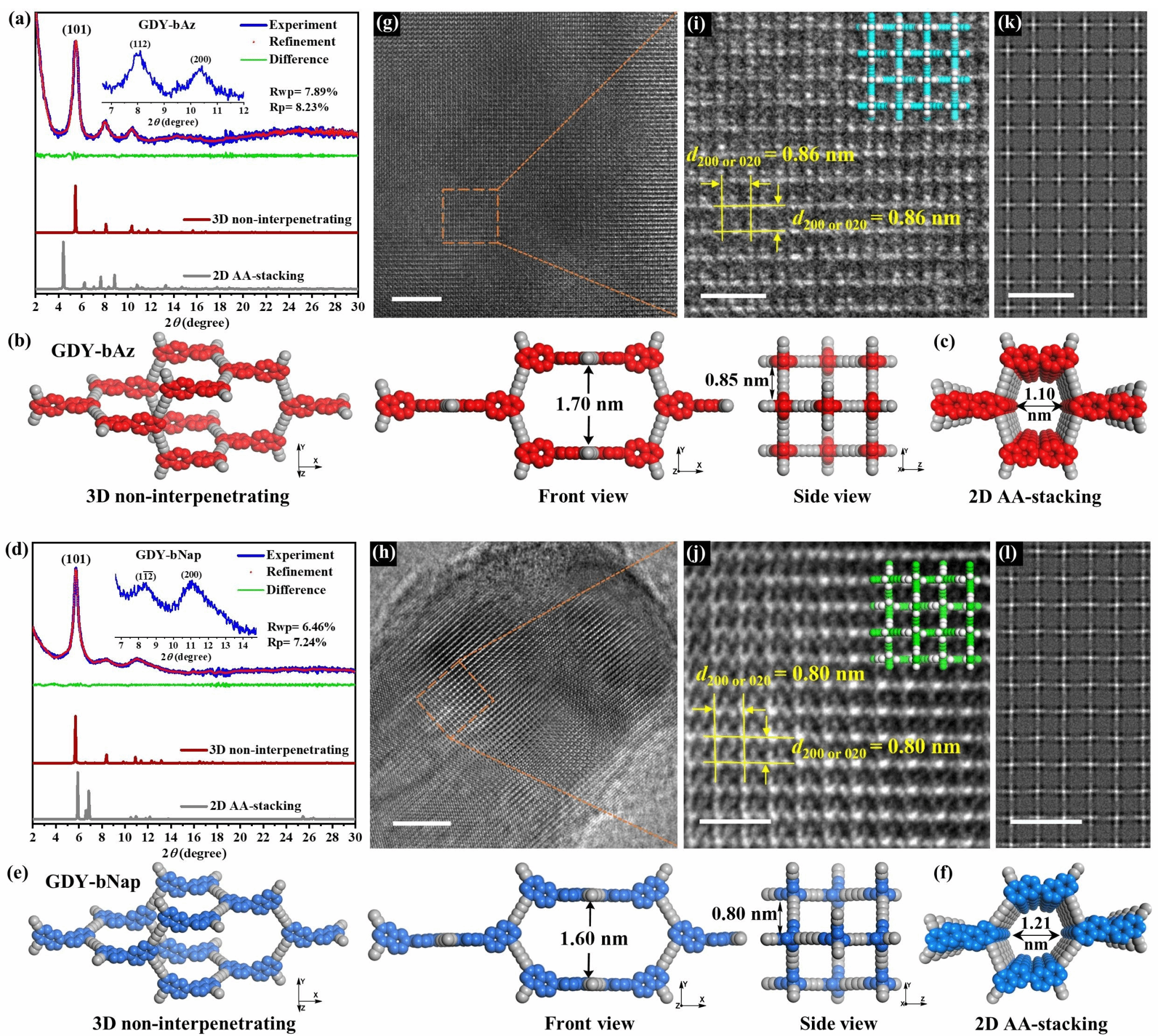
Figure 2. a) PXRD pattern of GDY-bAz. b) Simulated 3D structure of GDY-bAz, including a single adamantane cage and partial 3D frameworks in front and side views. c) Simulated 2D AA stacking model of GDY-bAz. d) PXRD pattern of GDY-bNap. e) Simulated GDY-bNap 3D structure, including a single adamantane cage and partial 3D framework views from front and side. f) Simulated GDY-bNap 2D AA stacking model. g) TEM image of GDY-bAz. h) TEM image of GDY-bNap. i) Magnified HR-TEM image of GDY-bAz showing square channels along the (001) direction, overlaid with the 3D structural model. j) Magnified HR-TEM image of GDY-bNap showing square channels along the (001) direction, overlaid with the 3D structural model. k) Simulated HR-TEM image of GDY-bAz. l) Simulated HR-TEM image of GDY-bNap.
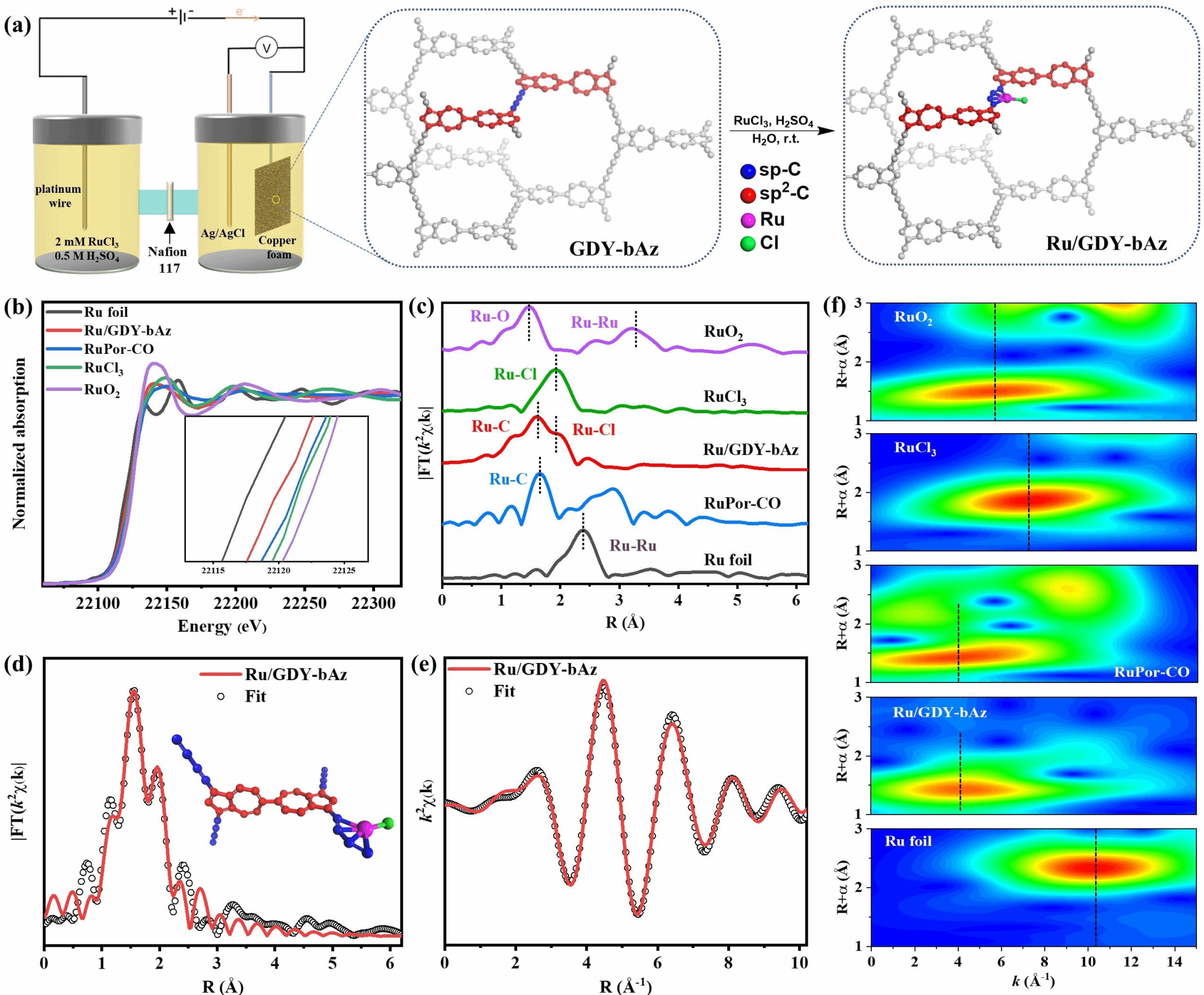
Figure 3. a) Synthesis of Ru/GDY-bAz on copper foam via direct electrochemical deposition in a three-electrode system. b) XANES spectrum of Ru/GDY-bAz at the Ru K-edge. c) Fourier-transformed k²-weighted χ(k) function EXAFS spectrum of Ru/GDY-bAz at the Ru K-edge. d) EXAFS fitting curve of Ru/GDY-bAz in R-space. e) EXAFS fitting curve of Ru/GDY-bAz in k-space. f) Wavelet transform of the k²-weighted EXAFS signal at the Ru K-edge for Ru/GDY-bAz.
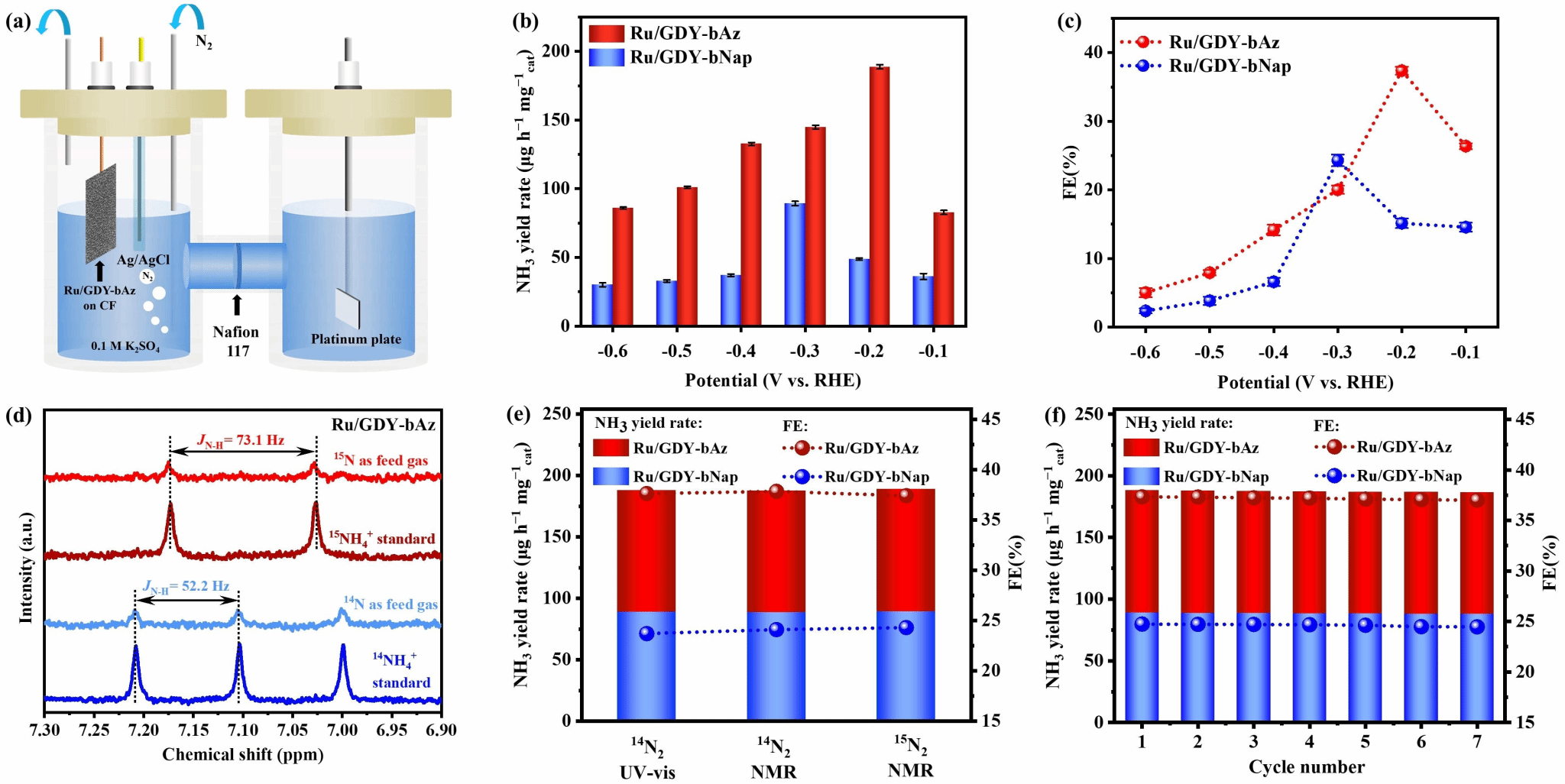
Figure 4. a) Electrolytic setup for ENRR. b) NH₃ yield of Ru/GDY-bAz and Ru/GDY-bNap at different potentials. c) Faradaic efficiency of Ru/GDY-bAz and Ru/GDY-bNap at different potentials. d) ¹H NMR spectra of ENRR products using different feed gases. e) Calculated NH₃ yield and Faraday efficiency of Ru/GDY-bAz at -0.2 V vs RHE and Ru/GDY-bNap at -0.3 V vs RHE based on NMR and UV-vis spectra. f) Cycling tests of Ru/GDY-bAz at -0.2 V and Ru/GDY-bNap at -0.3 V vs RHE.
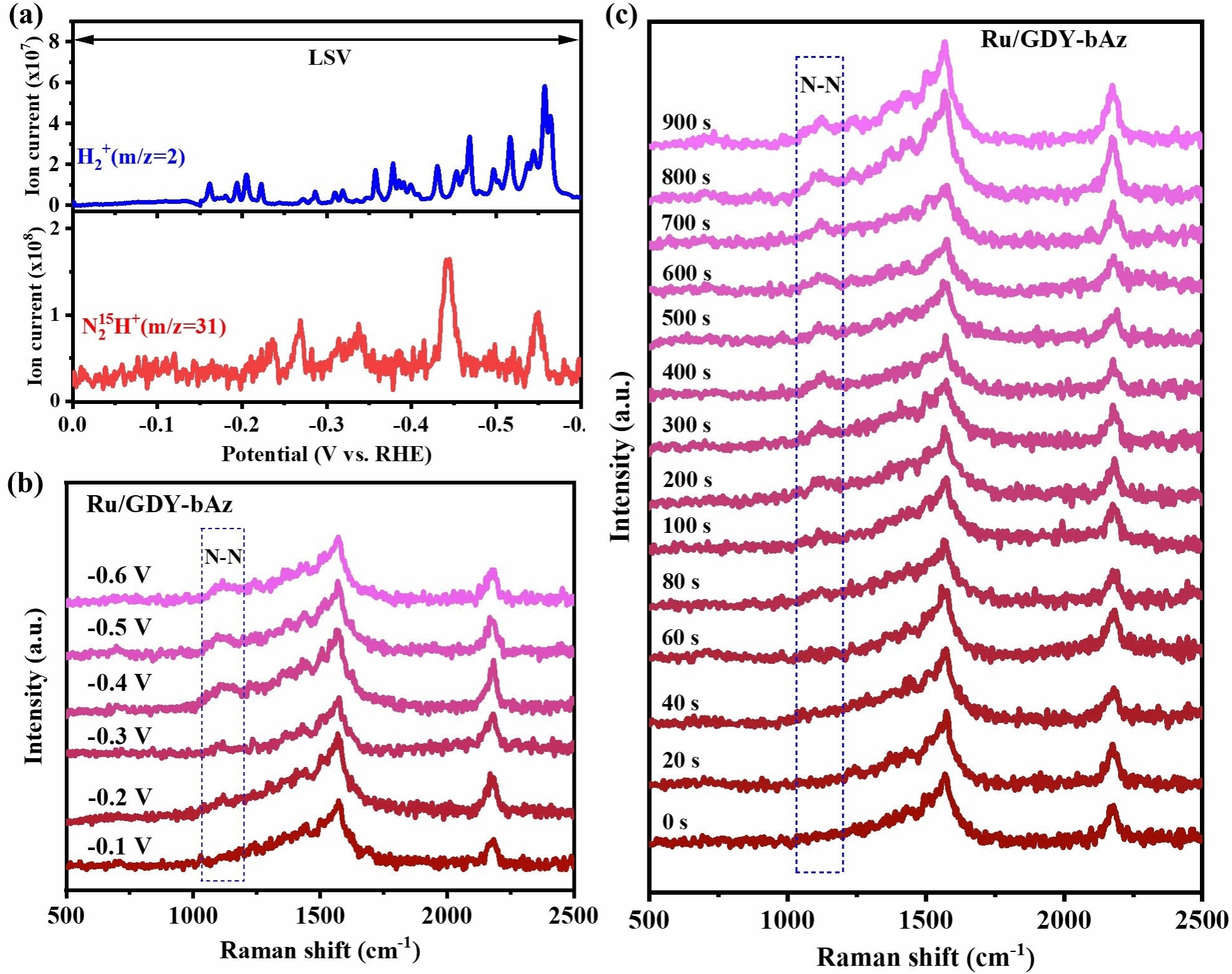
Figure 5. a) In situ DEMS data of Ru/GDY-bAz during scanning from 0 to −0.6 V vs RHE in N₂-saturated 0.1 M K₂SO₄ solution (H₂⁺ m/z = 2 and H⁺ m/z = 31). b) Potential dependence of N-N stretching on the Ru/GDY-bAz surface in N₂-saturated electrolyte, as measured by electrochemical in situ Raman spectroscopy. c) Time dependence of N-N stretching on the Ru/GDY-bAz surface in N₂-saturated electrolyte, as measured by electrochemical in situ Raman spectroscopy.
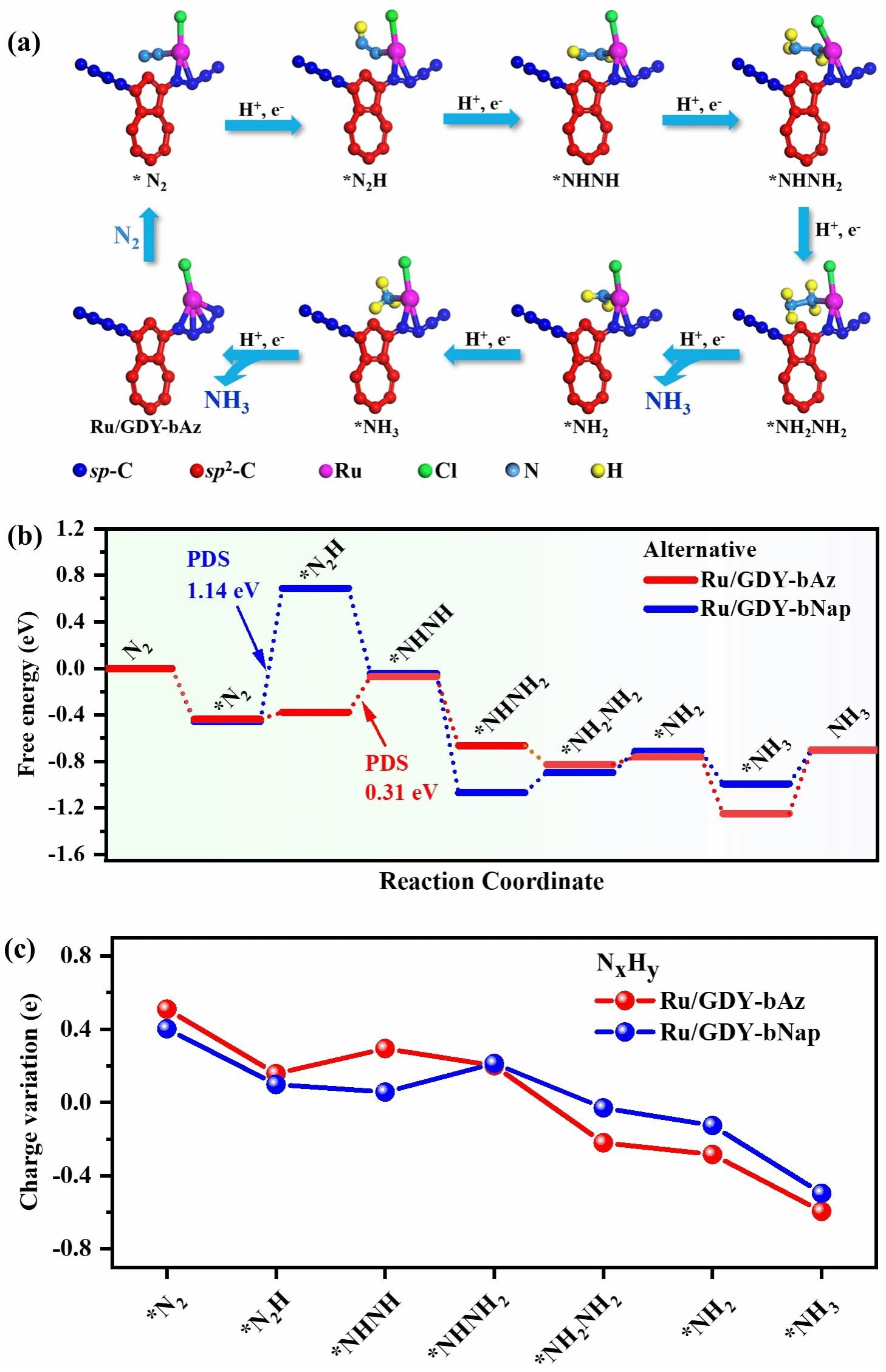
Figure 6. a) Catalytic cycle of the Ru/GDY-bAz surface alternating pathway. b) Free energy diagram of the ENRR alternating pathway on Ru/GDY-bAz and Ru/GDY-bNap. c) Bader charge changes of NxHy during the Ru/GDY-bAz and Ru/GDY-bNap-catalyzed ENRR alternating pathway.
Author: Team of Xiaodong Zhuang
Contributing Unit: Center for Innovation in Synthetic Science






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn