搜索


Recently, Nature Communications published online the research paper “A high-valence bismuth(V) nanoplatform triggers cancer cell death and anti-tumor immune responses with exogenous excitation-free endogenous H₂O₂- and O₂₂-independent ROS generation” by the team of Researcher Li Wanwan and Associate Professor Yu Xujiang from the Future Materials Innovation Center at Shanghai Jiao Tong University's Zhangjiang Advanced Research Institute. The paper describes a novel penta-valent bismuth nanomaterial (NaBiVO₃-PEG). This nanomaterial exhibits spontaneous reactive oxygen species (ROS) release behavior responsive to tumor microenvironment pH, independent of both exogenous excitation sources and endogenous tumor H₂O₂ and O₂, thereby inducing immunogenic cell death (ICD) and anti-tumor immune responses. The paper received high recognition from editors and was invited to contribute to the “Behind the Paper” section of Nature Portfolio Communities, sharing the research journey.
Most solid tumors harbor highly complex immunosuppressive microenvironments with low levels of pro-inflammatory immune cells, significantly limiting the clinical application of immunotherapy. ROS can directly induce tumor cell apoptosis and necrosis by damaging lipids, proteins, and DNA, while also triggering immune responses through immunogenic cell death. However, despite the effectiveness of various techniques—such as radiotherapy (RT), photodynamic therapy (PDT), and chemodynamic therapy (CDT)—in modulating ROS production, their reliance on exogenous X-rays or lasers and endogenous H₂O₂ or O₂ limits the development of efficient, convenient ROS-generating strategies and their clinical efficacy, thereby hindering effective tumor treatment. Identifying strategies that are less or not dependent on exogenous stimuli and endogenous H₂O₂ and O₂ levels is a current research focus in this field.
The research team demonstrated that pentavalent bismuth (BiV)-based nanomaterials can effectively address this issue (Figure 1). Upon cellular internalization, sodium bismuthate nanomaterials undergo sustained H+-accelerated hydrolysis. This process mediates the conversion of BiV to BiIII through electron transfer and releases lattice oxygen, generating abundant •OH and 1O2 radicals. This mechanism enables potent tumor immunotherapy.
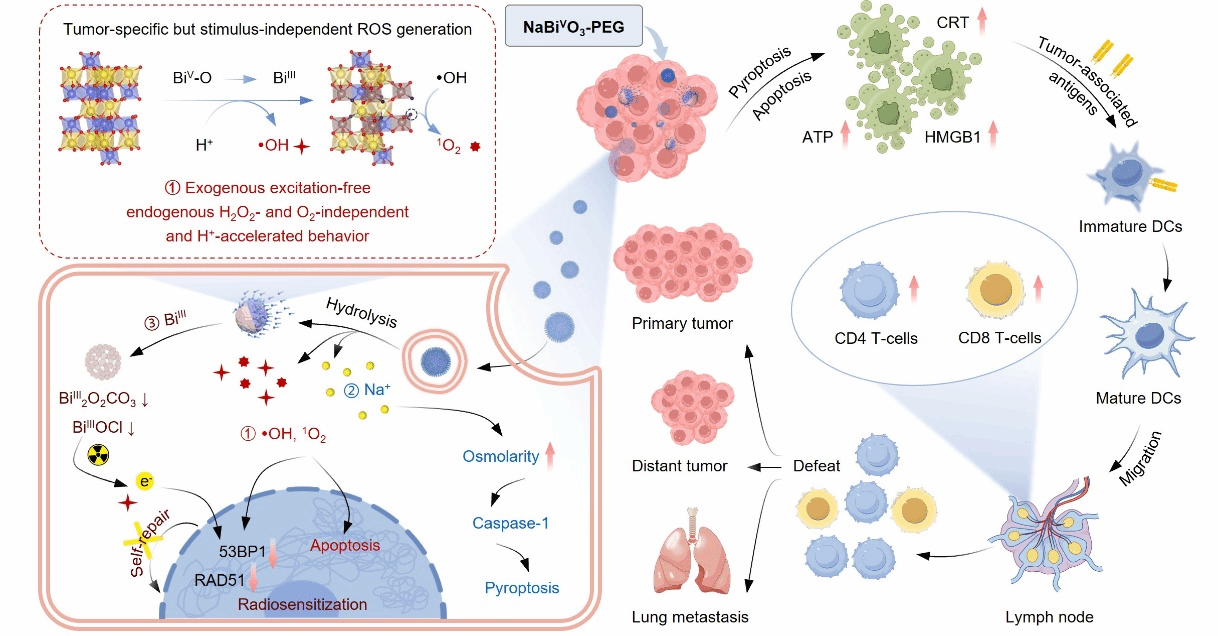
Figure 1. Schematic illustration of NaBiVO₃-PEG releasing •OH and 1O₂ under acidic conditions without exogenous stimulation after being internalized by tumor cells for tumor immunotherapy.
This study proposes a template-based approach using BiIIIOx nanoparticles as templates to etch and prepare uniformly porous, spherical, nanobranched-structured pentavalent bismuth (V) nanomaterials (NaBiVO₃-PEG). Surface modification with polyethylene glycol (PEG) phospholipid derivatives enhances dispersion in aqueous solutions (Fig. 2a and b). The resulting NaBiVO₃-PEG exhibits low hydrolysis and ROS generation under neutral conditions, yet demonstrates high reactivity in acidic buffers (pH 6.5–5.5) simulating tumor microenvironments or intracellular conditions. This accelerates spontaneous hydrolysis and generates abundant •OH and 1O₂ via electron transfer-mediated BiV-to-BiIII conversion without consuming ambient oxygen (Fig. 2c–f). Furthermore, the [BiO₆] octahedral lattice disintegrates during the BiV→BiIII conversion, releasing coordinated sodium ions (Figure 2g). Endogenous H₂O₂ further enhances the 1O₂ generation rate (Figure 2h). This work systematically demonstrates the hydrolysis behavior of pentavalent bismuth (V) materials and their ROS generation process without exogenous or endogenous stimulation—a phenomenon previously unreported.
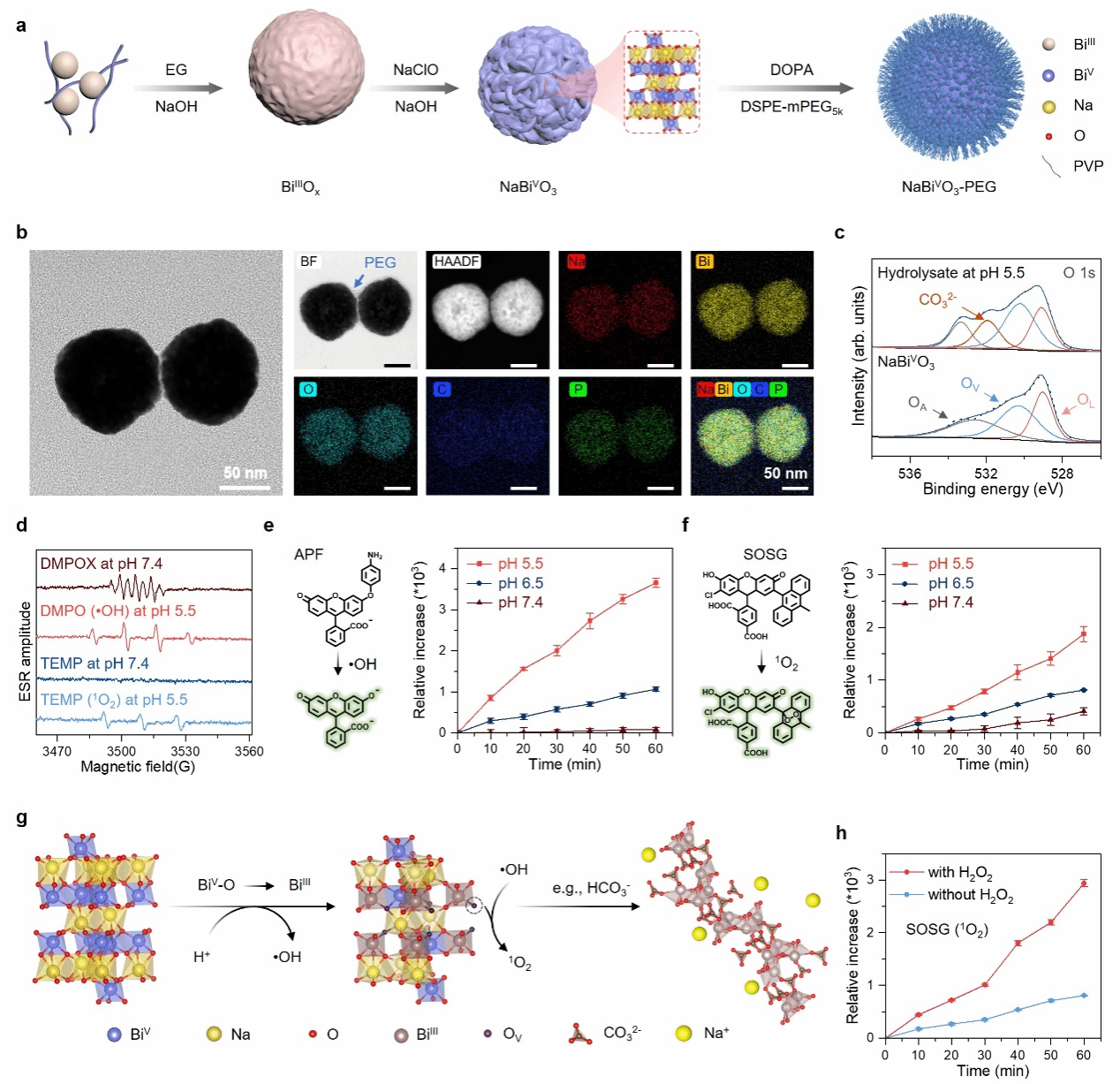
Figure 2. Preparation and characterization of NaBiVO₃-PEG: (a) Schematic of preparation; (b) TEM and elemental mapping images; (c) High-resolution XPS spectra of O¹⁻ after dispersion in SBF (pH 5.5) for 0 and 48 hours (OA: adsorbed oxygen, OV: oxygen vacancy, OL: lattice oxygen); (d) EPR spectrum; (e, f) •OH and 1O₂ release under different conditions; (g) Schematic of ROS formation mechanism; (h) 1O₂ release with and without H₂O₂ (1 mM).
Inspired by the specific ROS generation of NaBiVO₃-PEG in the tumor microenvironment, the study employed a 4T1 cell bilateral tumor mouse model and a lung metastasis mouse model to validate the immunotherapeutic and immunomemory mechanisms of NaBiVO₃-PEG in cancer treatment (Figure 3). Following intratumoral administration, NaBiVO3-PEG effectively suppressed primary tumors and enhanced immune system activation, increasing the infiltration ratio of CD4+ T helper cells and CD8+ cytotoxic T cells at the tumor site. Combined with Anti-CTLA4 immunotherapy, it effectively treated distant tumors, established immune memory, and inhibited tumor metastasis.
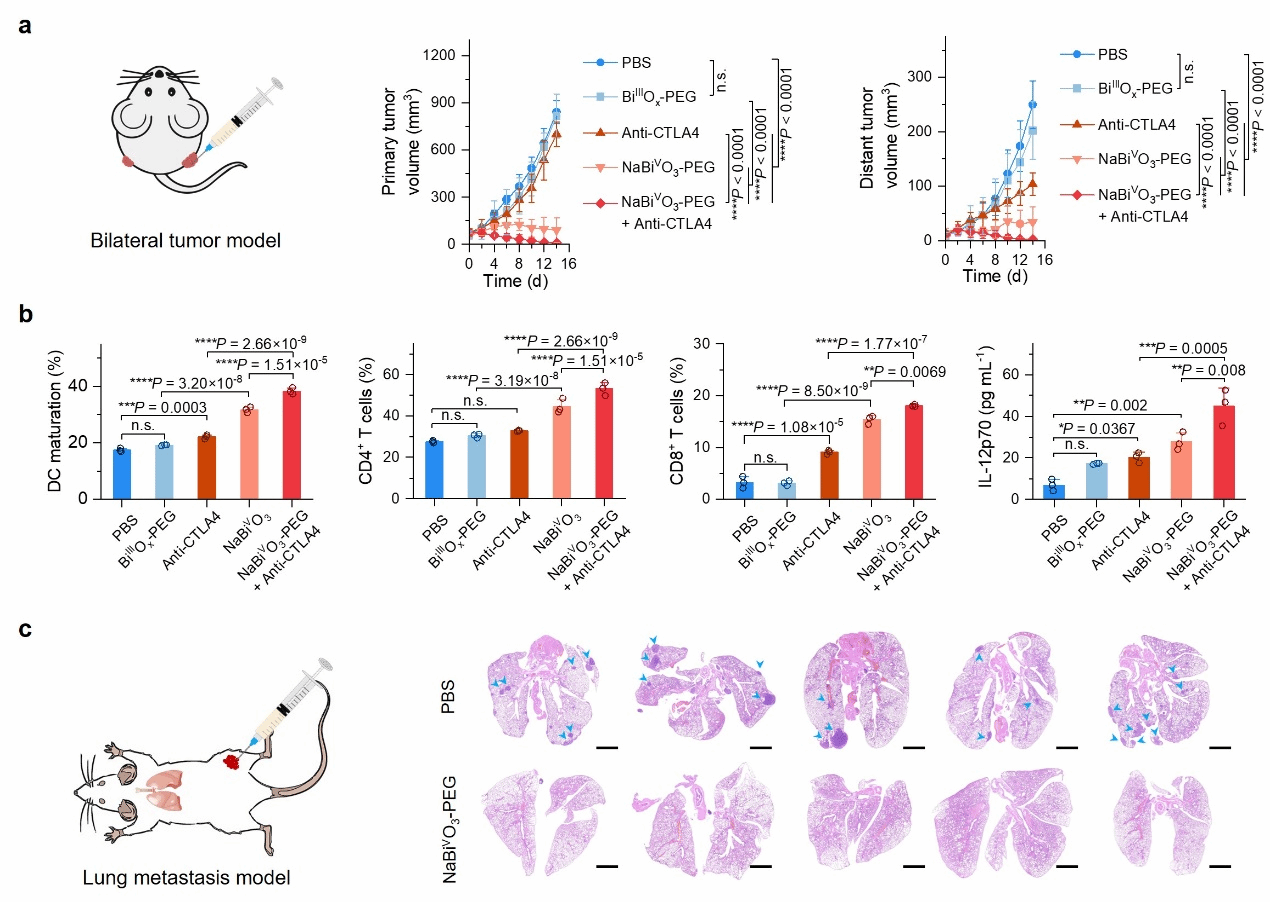
Figure 3. In vivo antitumor therapeutic effects of NaBiVO₃-PEG and corresponding immune responses. (a) Establishment of bilateral tumor mouse models and therapeutic efficacy; (b) Immune response analysis and immune cell infiltration in distant tumors; (c) Establishment of lung metastasis mouse models and therapeutic efficacy.
In summary, the research team reported and revealed the redox properties (BiIII→BiV→BiIII) of high-valent Bi(V) nanomaterials for cancer therapy—a characteristic previously unreported in studies involving bismuth-based nanomaterials. Concurrently, related work in organometallic and organic chemistry has explored the catalytic applications of Bi(V). Thus, this work inspires further design and application of Bi(V) materials through systematic mechanistic studies on the regulation of pentavalent bismuth nanomaterials. It also provides an efficient and feasible paradigm for tumor-specific ROS release via nanodrugs based on high-valent Bi(V).
Tang Yizhang, a doctoral candidate at the School of Materials Science and Engineering, Shanghai Jiao Tong University, is the sole first author of the paper. Li Wanwan, Researcher at the School of Materials Science and Engineering/Zhangjiang Advanced Research Institute, Shanghai Jiao Tong University, and Yu Xujiang, Associate Professor at the School of Materials Science and Engineering, are the co-corresponding authors. This research was supported by the National Natural Science Foundation of China (82372089, 82272823), the National Key R&D Program of China (2017YFA0205304), the Shanghai Translational Medicine Research Fund (TMSK-2021-117), and the Shanghai Natural Science Foundation (23ZR1434600).
Paper link: https://www.nature.com/articles/s41467-025-56110-7
Research sharing: https://communities.springernature.com/manage/posts/269712
Author: Li Wanwan Team
Contributing Unit: Center for Future Materials Innovation






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn