搜索


On January 24, 2025, The research group of Associate Professor Ni Jun from the School of Life Sciences and Technology at Shanghai Jiao Tong University and the Center for Innovation in Synthetic Science at the Zhangjiang Advanced Research Institute published a paper titled “Rational multienzyme architecture design with iMARS” in Cell. The study unravels the “code” linking spatial proximity and efficiency in multienzyme systems, resolving the longstanding challenge of lacking assembly standards for multienzyme complexes over the past 50 years. This breakthrough enables rational design of artificial multienzyme complexes from sequence to function.
Biocatalysis plays a pivotal role in cellular metabolism and biomanufacturing, with multienzyme cascade catalysis widely applied in pharmaceuticals, food processing, and industrial sectors. In nature, the spatial structure of multienzyme complexes is often finely tuned to regulate catalytic reaction rates. Inspired by nature, researchers have employed diverse assembly strategies—including fusion enzymes, protein scaffolds, and nucleic acid scaffolds—to construct artificial multienzyme complexes, thereby enhancing the catalytic efficiency of cascade reactions. However, the spatial structure-function relationship of artificial multienzyme complexes has perplexed the academic community for over five decades. Artificial multienzyme assembly has relied solely on experimental trial and error, lacking rational design tools, which has limited its applications.
To address these challenges, the research team deciphered the spatial structure-function relationship of artificial multienzyme complexes through high-throughput testing and spatial structure prediction. They preliminarily cracked the “code” linking multienzyme spatial proximity and efficiency, identifying the spatial distance between fusion enzymes and channel angles as key factors influencing catalytic efficiency. Building on these findings, the team developed iMARS, a rational design tool for artificial multienzyme complexes. This tool incorporates a database of thousands of linkers with diverse properties, employs ParaFold for high-throughput protein structure prediction, and utilizes computational methods like molecular docking and CAVER alongside DO Score evaluation for screening. This enables rapid prediction of relative activity in artificial multienzyme complexes directly from amino acid sequences.
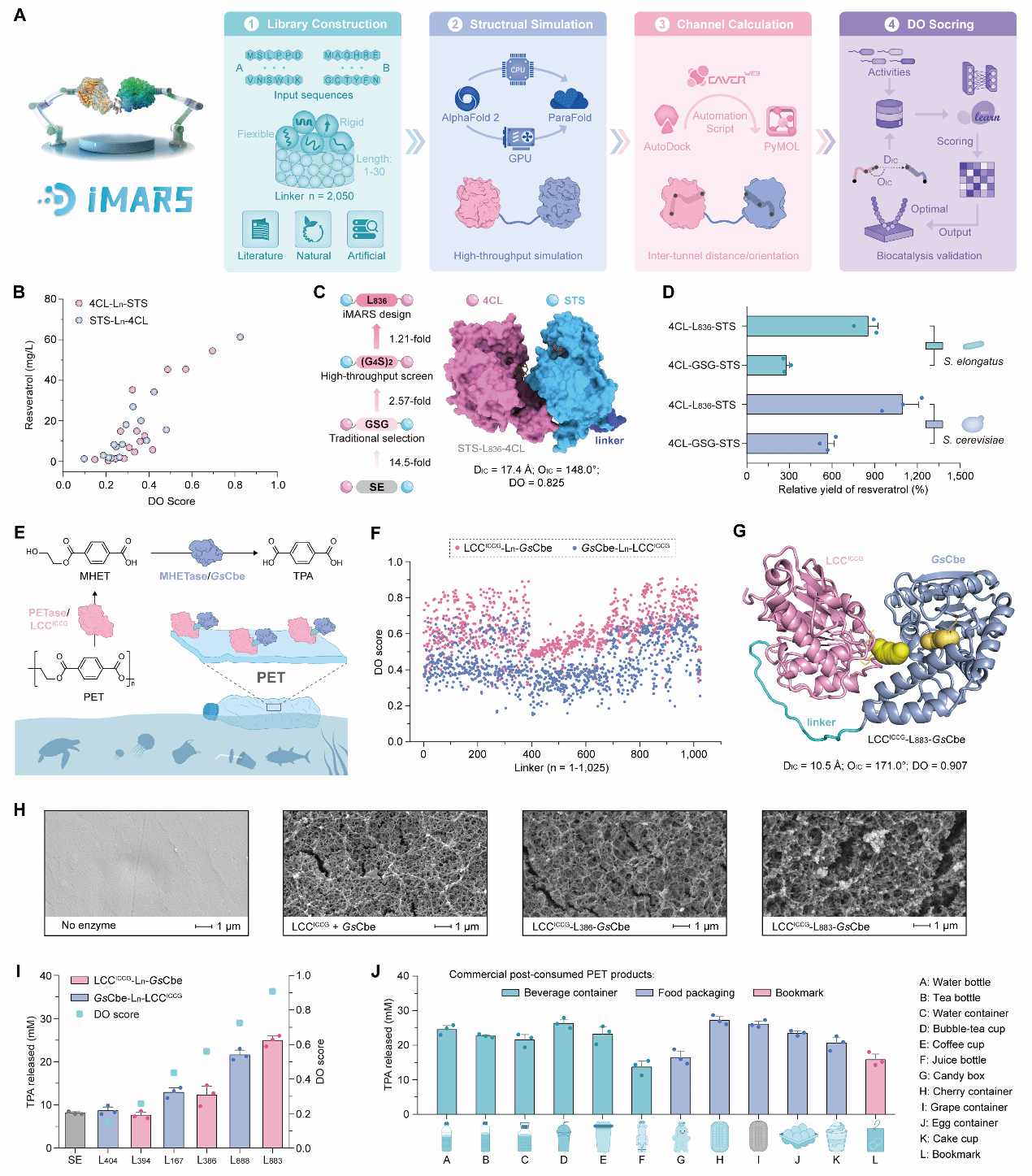
The research team explored the design capabilities of the iMARS method across diverse applications, including the biosynthesis of compounds such as resveratrol, vanillin, and ergothioneine, as well as the biodegradation of PET plastics. Specifically, the engineered fusion enzyme achieved a 45.1-fold increase in resveratrol production in engineered E. coli compared to free enzymes. Its application in Saccharomyces cerevisiae and the photosynthetic microorganism cyanobacteria demonstrated iMARS's broad applicability as a molecular-level design tool across diverse host cells. In vitro PET biodegradation experiments revealed more pronounced degradation patterns on PET films when observed via scanning electron microscopy (SEM) using the engineered fusion enzyme. During batch fed-batch fermentation for ergothioneine synthesis, engineered strains incorporating the optimized fusion enzyme achieved peak production efficiency, demonstrating iMARS' potential for industrial biomanufacturing. Currently, iMARS is available online (http://www.imars-lumybio.com/), providing researchers with a convenient platform for rapid multi-enzyme complex design.
This study, developed using multiple deep learning software tools, exemplifies the advancement of artificial intelligence in biology. The process of “creating, learning, and designing” multi-enzyme complexes embodies the synthetic biology philosophy of “creating to gain knowledge and creating for practical application.” The iMARS tool developed in this study is the first rational design tool for artificial multi-enzyme complexes. It marks a new era in multi-enzyme assembly, transitioning from reliance on experimental trial-and-error to rational design. This advancement will accelerate the development of synthetic biology and biomanufacturing, holding significant implications for pharmaceuticals, food, and industrial sectors.
Associate Professor Ni Jun from the School of Life Sciences and Technology, Shanghai Jiao Tong University; the State Key Laboratory of Microbial Metabolism; and the Center for Synthetic Science Innovation at Zhangjiang Advanced Research Institute served as corresponding author. Wang Jiawei, a doctoral candidate at Shanghai Jiao Tong University, is the first author, with Shanghai Jiao Tong University listed as the primary institution. Professor Zhao Yilei from Shanghai Jiao Tong University and Meng Shiyu from Shanghai Guangyue Biotechnology Co., Ltd. provided crucial support for this research. This research was supported by the National Natural Science Foundation of China, the National Key R&D Program of China, and Guangyue Biotech.
Paper link: https://www.cell.com/cell/fulltext/S0092-8674(24)01471-5
Author: Jun Ni's Team
Contributing Unit: Center for Innovation in Synthetic Science






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn