搜索


Electrochemical conversion of carbon dioxide (CO₂) into higher-value chemical products using renewable electricity represents one of the most promising technologies for achieving carbon neutrality. Among numerous products, ethylene holds immense industrial demand as a feedstock for diverse chemical derivatives via catalytic processes. Within electrochemical CO₂ reduction, C-C coupling is recognised as the pivotal step for ethylene formation. Cu-based catalysts exhibit high selectivity for ethylene production due to moderate adsorption energies for *CO intermediates on Cu surfaces. To enhance the selectivity of Cu-based catalysts, researchers have reported numerous effective regulation strategies, including control of grain boundaries, oxidation states, doping, morphology, and surface microenvironments. Despite the extensive reporting of highly active Cu-based catalysts, achieving high selectivity at high current densities remains a significant challenge.
Recently, collaborative research by Professor Zhuang Xiaodong's group at Shanghai Jiao Tong University, Professor Wang Tianfu's group, and Professor Zhang Juan's group at the University of Science and Technology Beijing has reported a molecular dipole regulation strategy to enhance CO₂ adsorption on the Cu surface, thereby boosting electrocatalytic CO₂ reduction activity. Gaussian calculations revealed that 2-aminoindole, featuring a five-membered ring-seven-membered ring topology, exhibits a larger dipole moment than aromatic structures such as aniline and naphthylamine (2-aminoindole: 2.31 D; aniline: 1.64 D; naphthylamine: 1.96 D). To investigate the influence of dipole moment on CO₂ adsorption and electrocatalytic performance, the team synthesised 2-aminoazulene as a monomer with a large dipole moment. Polyazulene (PAAz) was prepared via oxidative polymerisation. Subsequently, Cu nanoparticles were loaded onto the PAAZ surface using wet chemical and electro-reduction methods, yielding the Cu@PAAz composite catalyst. Molecular dynamics simulations and CO₂ gas adsorption results demonstrated that PAAZ exhibits superior CO₂ adsorption capacity compared to polyaniline and poly(naphthylamine). Employing Cu@PAAz as an electrocatalyst for CO₂ reduction yielded an ethylene Faraday efficiency of 68.9% at a high current density of 1 A cm⁻², significantly surpassing the performance of bare Cu catalysts. In situ Raman spectroscopy and in situ Fourier transform infrared spectroscopy revealed higher concentrations of *CO intermediates at the Cu/PAAz interface compared to bare Cu. This is primarily attributed to hydrogen bonding interactions between the amine groups on PAAz and the *CO intermediates, thereby achieving chemical stabilisation. Theoretical calculations indicate that PAAZ significantly reduces the energy barrier for C–C dimerisation, effectively suppressing hydrogen evolution reactions on the Cu surface and thereby promoting the electrocatalytic conversion of CO₂ to ethylene. This study not only deepens the understanding of the influence of dipole moment on CO₂ RR performance but also provides a novel approach for preparing highly active catalysts.
The work is currently published online in Advanced Materials under the title ‘Large Dipole Moment Enhanced CO₂ Adsorption on Copper Surface: Achieving 68.9% Catalytic Ethylene Faradaic Efficiency at 1.0 A/cm²’ (Adv. Mater. 10.1002/adma.202415092). This research received funding from the National Natural Science Foundation of China, the Shanghai Municipal Science and Technology Commission, and the China Postdoctoral Science Foundation.
Original article link: https://doi.org/10.1002/adma.202415092
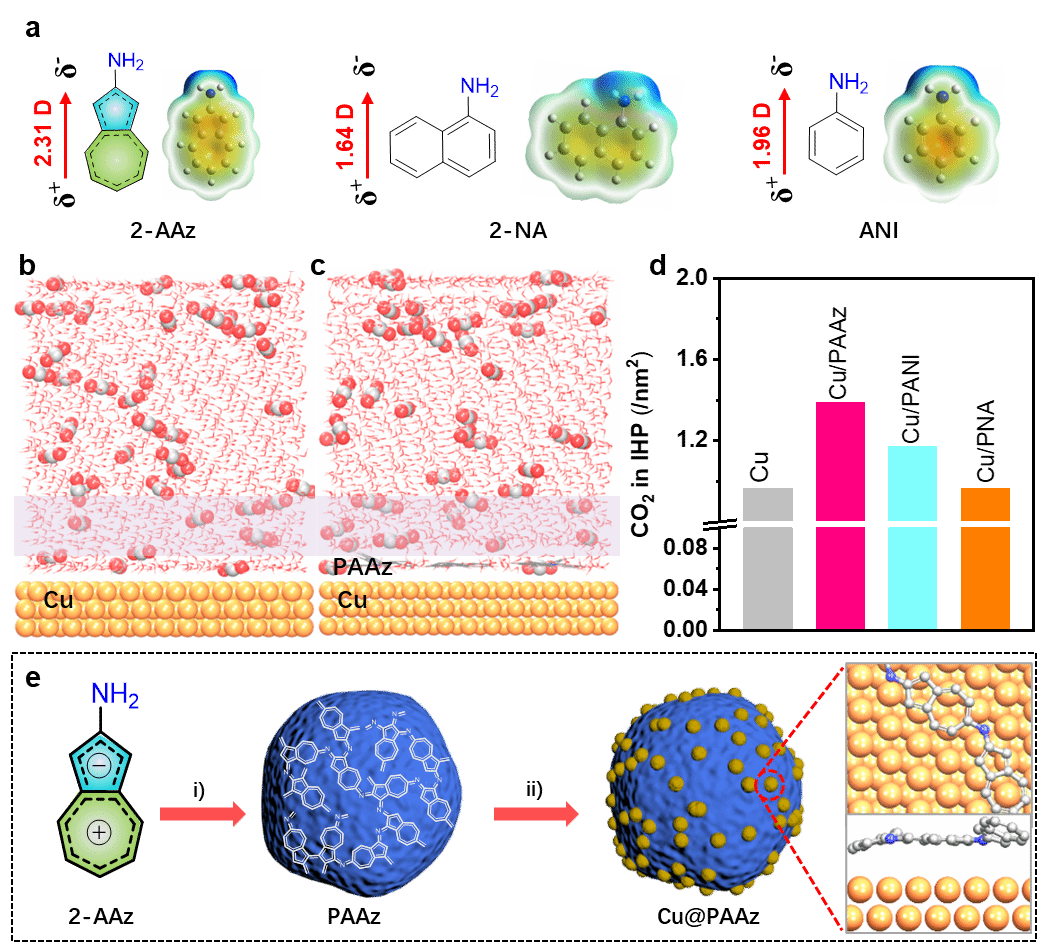
Figure 1. a) Calculated dipole moments and electric potentials for Az, 2-AAz, 2-NA and ANI; blue and yellow denote positive and negative charges respectively. Molecular dynamics-simulated interface structures: b) water/Cu interface, c) water/PAAz/Cu interface. d) Comparison of local CO₂ concentrations on Cu, Cu/PAAz, Cu/PANI and Cu/PNA surfaces. e) Schematic of the Cu@PAAz preparation process.
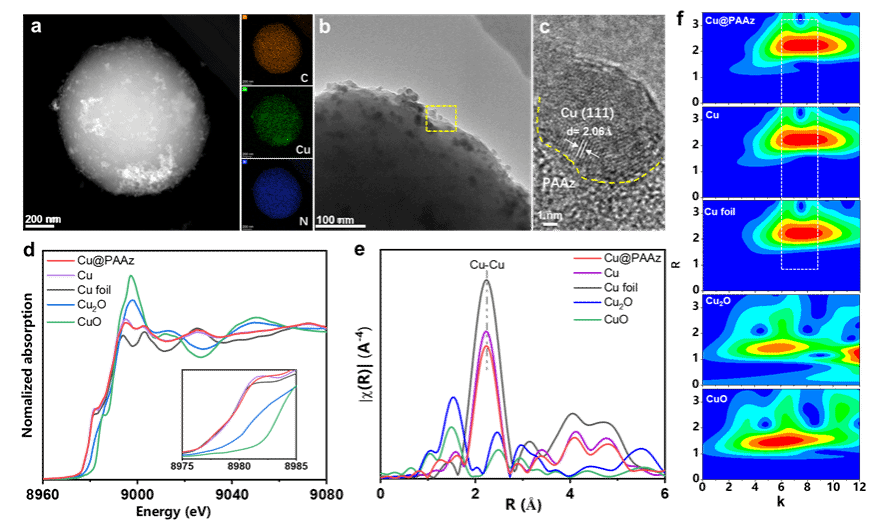
Figure 2. a) HAADF-STEM image of Cu@PAAz. b, c) High-resolution TEM images of Cu@PAAz. d) Cu K-edge XANES spectra for Cu@PAAz, Cu, Cu foil, Cu₂O, and CuO. e) FT-EXAFS spectrum. f) Wavelet transform.
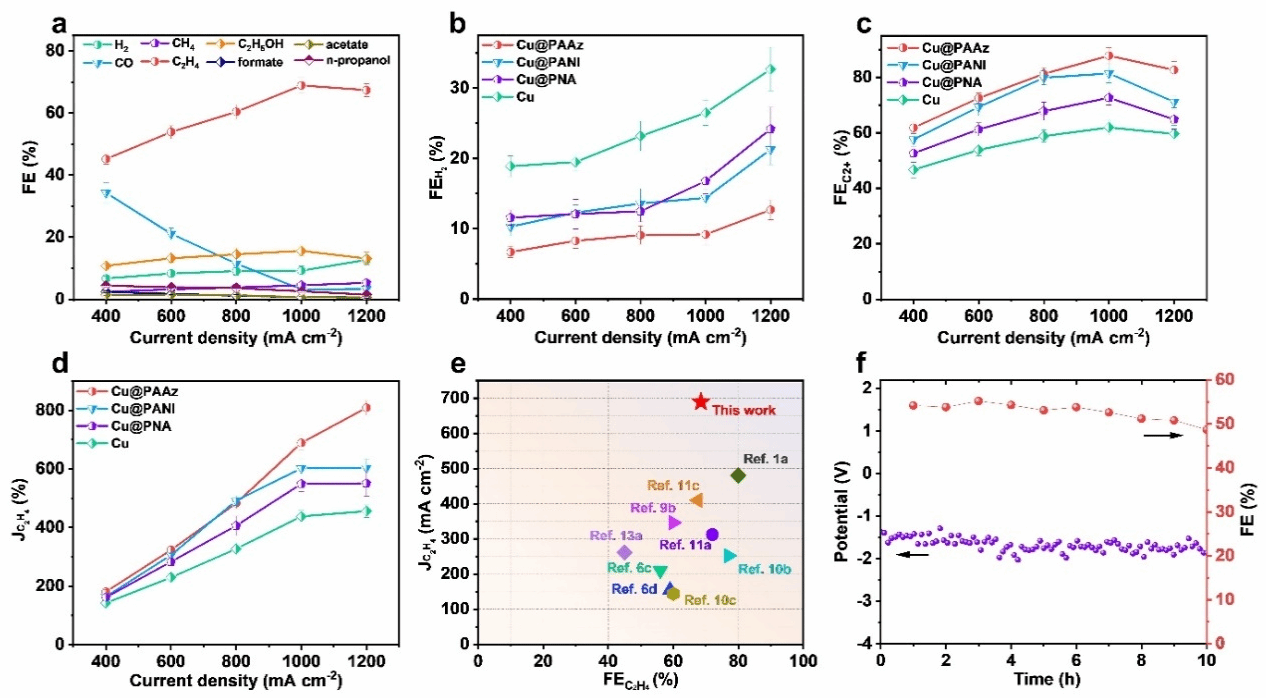
Figure 3. a) Faradaic efficiencies of all products from Cu@PAAz at different current densities. b) Faradaic efficiency for H₂ and c) C₂⁺ products from Cu@PAAz, Cu@PNA, Cu@PANI and Cu. d) Ethylene partial current density for Cu@PAAz, Cu@PNA, Cu@PANI and Cu. e) Performance comparison with previously reported catalysts. f) Long-term cycling stability.
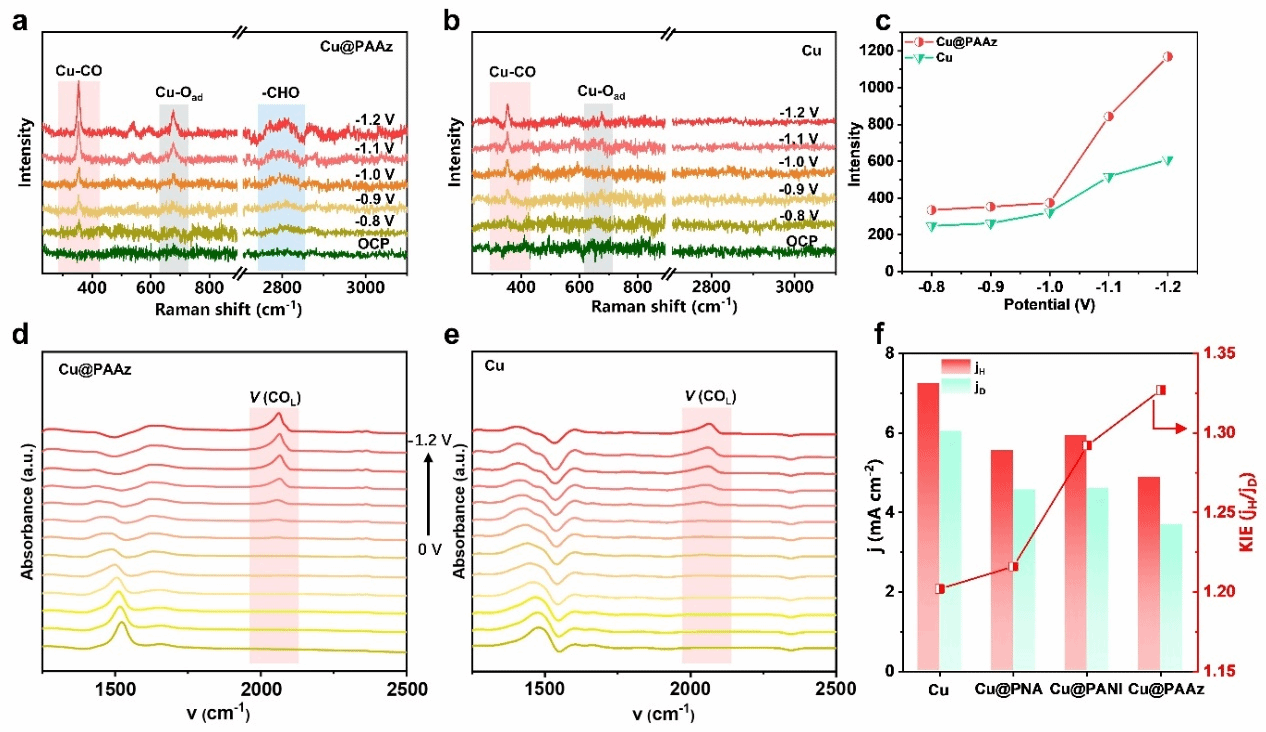
Figure 4. a) and b) In situ Raman spectra. c) Comparison of Cu-CO signal intensity on Cu@PAAz and Cu electrode surfaces. d) and e) In situ infrared spectra. f) KIE curves measured for Cu@PAAz, Cu@PNA, Cu@PANI, and Cu electrodes.
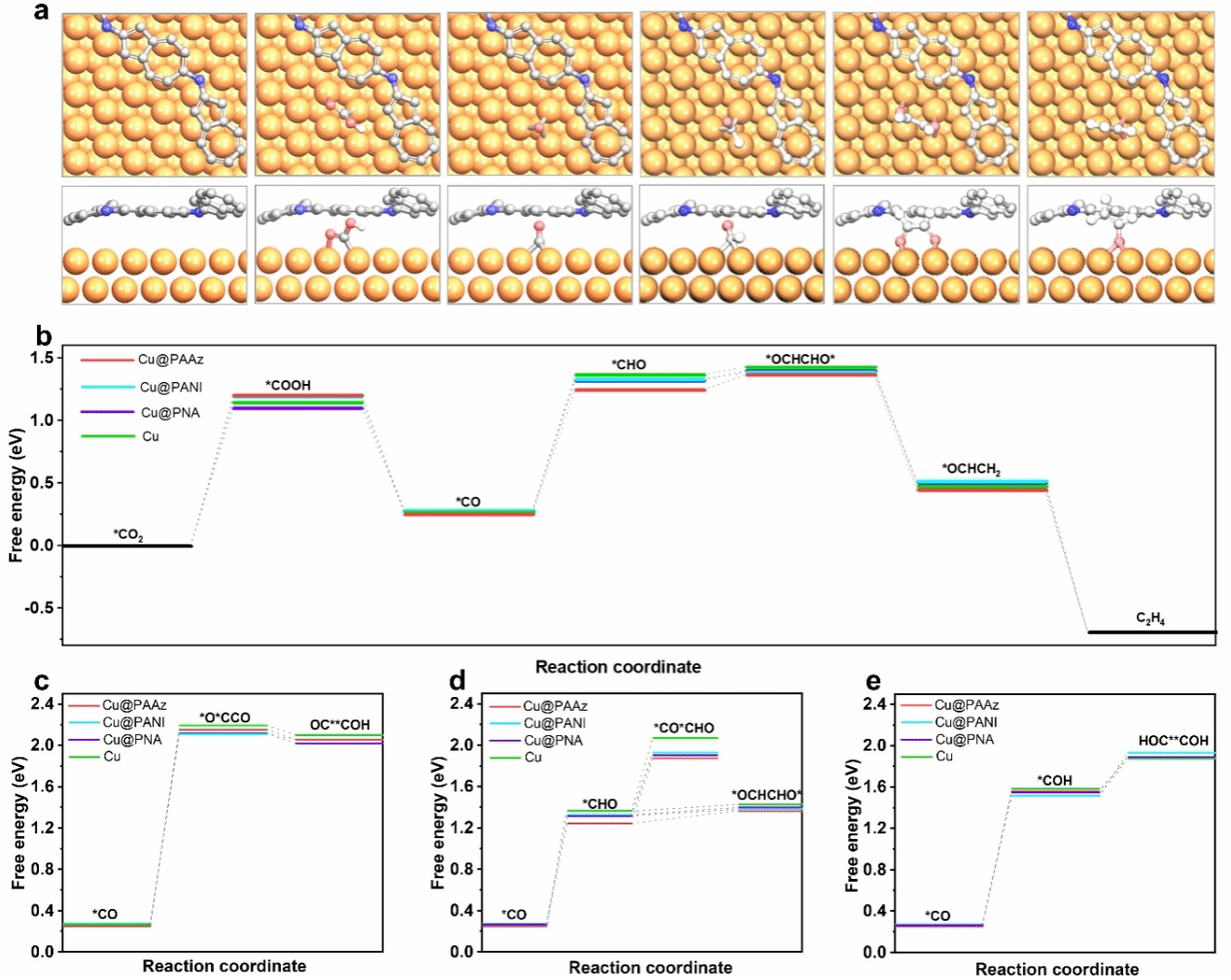
Figure 5. a) Theoretical simulation of the CO₂ reduction reaction process. b) Gibbs free energy for CO₂ reduction to C₂H₄. c), d), e) Gibbs free energy for different CO dimerisation reaction pathways on various Cu surfaces.
Authors: Zhuang Xiaodong's research group
Contributing institution: Centre for Innovation in Synthetic Science






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn