搜索


Recently, Wu Jianbo and Gao Wenbai from the Atomic Manufacturing Centre at the Future Materials Innovation Centre, Zhangjiang Advanced Research Institute, Shanghai Jiao Tong University (School of Materials Science and Engineering), alongside Deng Tao from the School of Materials Science and Engineering, in collaboration with Professor Pan Xiaoqing from the University of California, Irvine, achieved a breakthrough in the atomic-scale manipulation of surface ordering in platinum-based alloy nanowires. Their research findings, titled ‘Highly Stable and Active Catalyst in Fuel Cells Through Surface Atomic Ordering’ (DOI: 10.1126/sciadv.ado4935) was published in the premier international materials science journal Science Advances, with Shanghai Jiao Tong University listed as the first author institution. By restricting atomic diffusion in the surface layer, the research team facilitated phase transitions while preserving shape integrity, achieving atomic ordering transformations in platinum-iron nanowires at low temperatures. This innovative strategy significantly enhances catalyst stability within membrane electrode assemblies (MEAs). Surface atomic ordering reduces loss of active metals while maintaining high catalytic activity comparable to that observed in half-cells. Employing in-situ scanning transmission electron microscopy (STEM), the team elucidated optimal annealing conditions for inducing surface atomic ordering in one-dimensional platinum-iron nanowires (PtFe NWs) while preserving their morphology. Findings indicate that surface atomic diffusion and ordering occur at temperatures significantly below the bulk phase transition temperature. This low-temperature condition suppresses atomic motion within the nanowire interior, preventing fracture and sintering of the one-dimensional morphology. The paper's first author is Dr. Ma Yanling from the School of Materials Science and Engineering at Shanghai Jiao Tong University. Co-corresponding authors include Professors Wu Jianbo and Gao Wenbai from the Centre for Future Materials Innovation at the Zhangjiang Advanced Research Institute, Shanghai Jiao Tong University, Professor Deng Tao from the School of Materials Science and Engineering, and Professor Pan Xiaoqing from the Department of Materials Science and Engineering at the University of California, Irvine, served as co-corresponding authors. The research received substantial support from Associate Researcher Shang Wen, Associate Professor Song Chengyi, Professor Tao Peng from the School of Materials Science and Engineering at Shanghai Jiao Tong University, and Professor Zhu Hong from the University of Michigan. Shanghai Jiao Tong University is listed as the first completing institution.
Proton exchange membrane fuel cells (PEMFCs) represent a highly promising energy conversion device. However, the slow oxygen reduction reaction (ORR) at the cathode adversely affects PEMFC efficiency, power density, and operational lifetime. To address these challenges, various nanomaterials based on the noble metal platinum (Pt) have been developed. Among these, shape-controlled alloy nanocrystals have demonstrated outstanding activity and stability in rotating disk electrode (RDE) testing. Nevertheless, the stringent operating conditions within fuel cells—including continuous potential fluctuations, elevated operating temperatures, and high current densities—may precipitate rapid leaching and degradation of metallic catalysts within membrane electrode assemblies (MEAs). Furthermore, leached metal cations within the MEA can accumulate near the surface, impeding proton transport. Therefore, enhancing the stability of morphology-controlled Pt-based alloy ORR catalysts is crucial for their effective application in the cathode layer of PEMFCs.
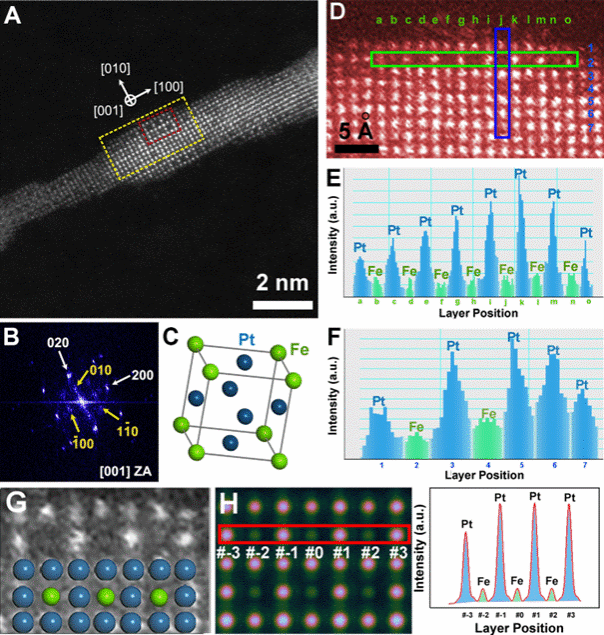
Figure 1. Structural characterisation of ordered phases on PtFe nanowire surfaces under low-temperature annealing conditions. A, HAADF-STEM image of PtFe nanowires annealed at 350°C for 30 minutes. B, Surface region FFT map of the superlattice site in Figure 1A, showing the A1-PtFe solid solution phase (marked by white arrows) and the ordered L12-Pt3Fe phase (marked by yellow arrows). C. Unit cell of the ordered L12-Pt3Fe structure. Pt atoms are blue, Fe atoms are green. D. Colour-enhanced magnified image cropped from the yellow box in Figure 1A. E–F. Integral intensity distribution maps corresponding to atomic layers in the green and blue rectangular regions of Figure 1D. G. Magnified lattice and corresponding atomic model cropped from the red box in Figure 1A. H. HAADF-STEM image of the L12-Pt3Fe model and integral intensity distribution map of the atomic layer within the red rectangle.
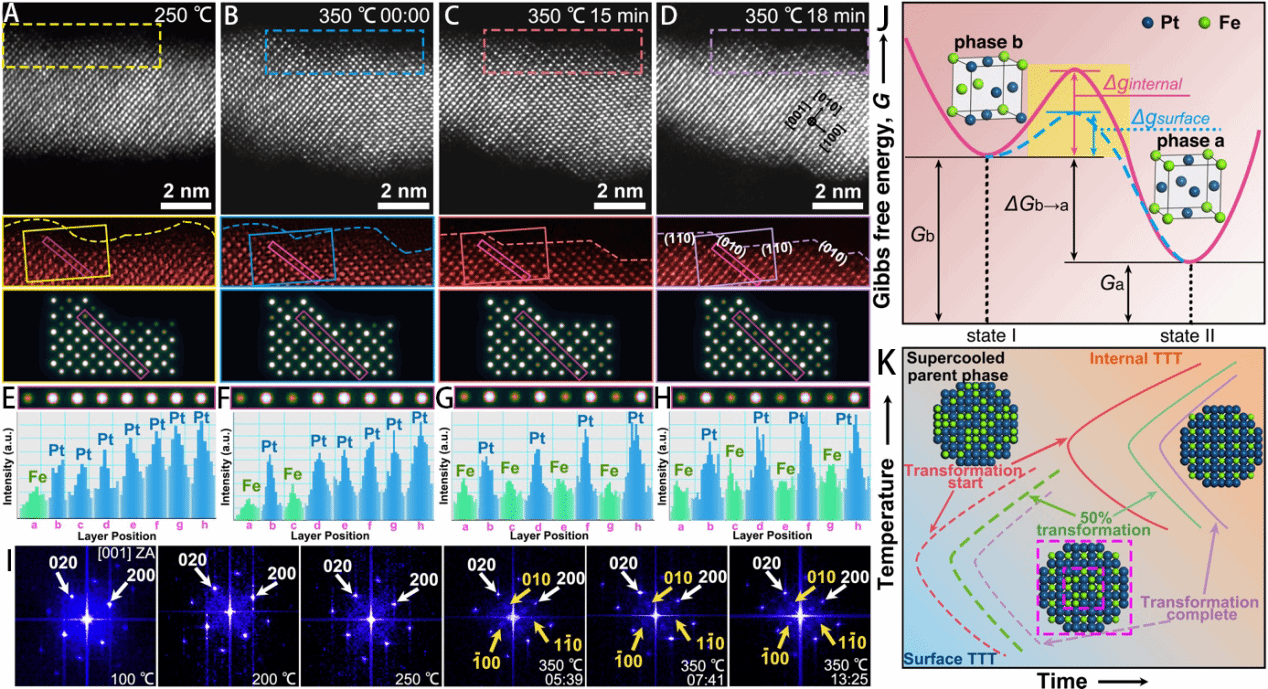
Figure 2. Disorder-to-order transition in the surface structure of PtFe nanowires. A–D, HAADF-STEM images after heating at 250 °C, 350 °C for 0 minutes, 350 °C for 15 minutes, and 350 °C for 18 minutes. E–H, Integrated intensity distributions along atomic layers for Figures 2A–D. I, FFT maps corresponding to surface regions at different temperatures or after varying annealing times at 350 °C. J, Schematic energy barrier for the phase transition from the b phase to the a phase. K, TTT maps for surface and bulk regions, illustrating phase transition kinetics from the undercooled parent phase to the ordered phase.
To investigate the disorder-to-order transition in PtFe alloys, researchers synthesised 3 nm PtFe alloy nanowires in the solid state. These were dispersed on a heated substrate for in situ STEM imaging, with 30-minute dwells at 50°C increments. Figure 1 demonstrates that nanowire morphology remained unchanged at 350°C, exhibiting no aggregation or fracture, and confirms the formation of intermetallic compounds on the PtFe nanowire surface following low-temperature annealing. Figure 2 depicts the structural evolution of the nanowires at varying temperatures and holding times to determine the kinetics of surface atomic ordering at low temperatures. The near-surface region of PtFe nanowires remained in the disordered phase at 250°C. Upon heating to 350°C and holding for 15 minutes, nearly the entire surface transformed into the Ll2-Pt3Fe phase, exhibiting distinct step-like features on the ordered surface after 18 minutes. However, annealing times exceeding half an hour or temperatures as high as 500°C and 600°C caused PtFe nanowires to fracture. Through the aforementioned in situ STEM observations, we determined the optimal annealing conditions for inducing surface atomic ordering in one-dimensional PtFe nanowires while preserving their morphology: a temperature capped at 350°C and a holding time not exceeding half an hour.
This surface atomic ordering occurs at low temperatures due to size effects inducing thermodynamic and kinetic differences in the phase transition process. Smaller materials exhibit lower energy barriers, enabling atomic ordering at reduced temperatures. In this work, nanowires with diameters of only a few nanometres further lower the temperature required for phase transition. Atomic ordering is thermodynamically driven, with the phase transition motivated by the Gibbs free energy difference ΔGb-a between phases, which depends on the annealing temperature. However, initiating lattice reconstruction necessitates overcoming an additional energy barrier Δg arising from interatomic attractive forces. Weak interactions between low-coordination atoms and surface-proximal atoms can further diminish Δg, whilst surface atoms exhibit greater mobility due to heightened thermal vibrations. Consequently, a pronounced distinction exists between surface and bulk regions for atomic ordering in nanomaterials, with the lower energy barrier in surface areas promising to facilitate surface atomic ordering and phase segregation.
Furthermore, considering surface-preferred nucleation, the time-temperature transition (TTT) curve for the surface region may occur within the low-temperature zone, with the time scale for completing surface atomic ordering being relatively short (Figure 2K). It should be noted that although thermodynamics permits transitions to occur at the surface at low temperatures, the migration of phase boundaries between the newly formed ordered phase and the solid solution phase is hindered due to slow atomic diffusion within the nanowires. Consequently, phase transition kinetics slow considerably as one moves into the interior. Consequently, after annealing at 350°C for 30 minutes, ordered phases in PtFe nanowires were observed only within a thickness not exceeding three unit cells. In this study, despite an overall Pt:Fe atomic ratio close to 1, an ordered Pt₃Fe L12 phase formed rather than a PtFe L10 phase. This arises because surface segregation of Pt atoms and lattice ordering induced by atomic diffusion occur simultaneously. The ordering of surface atoms is not driven by compositional segregation, as the disorder-to-order phase transition temperature is significantly higher than the temperature employed (350°C) – occurring at 1300–1350°C for bulk Pt-Fe and >550°C for Pt-Fe nanomaterials. Consequently, this low-temperature surface atomic ordering (LT-SAO) approach holds promise for application to other Pt-based alloy nanocrystals while simultaneously preserving their complete morphology.
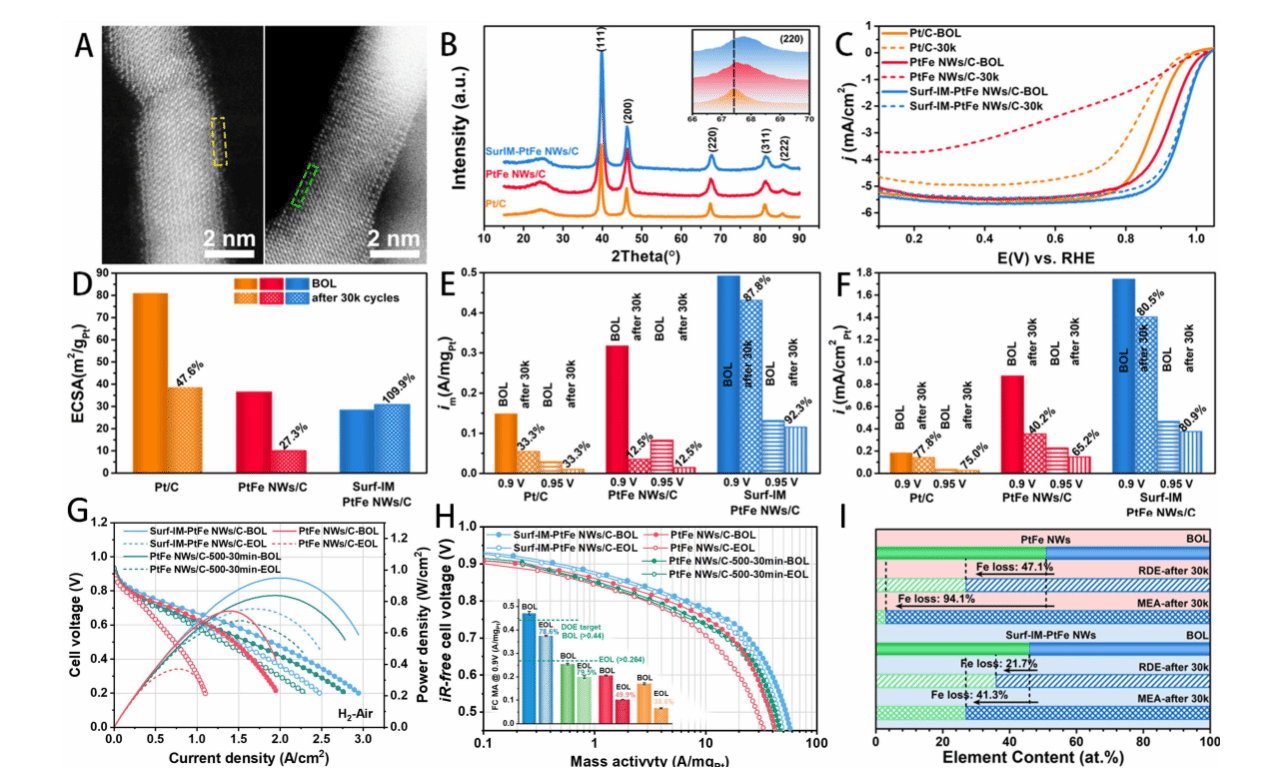
Figure 3. Investigation of oxygen reduction performance (A–F) and fuel cell performance (G–I) of PtFe nanowires
As shown in Figure 3, the high catalytic activity of the Surf-IM-PtFe NWs catalyst, evaluated via RDE measurements, is similarly reflected in fuel cell test results. Compared to completely random PtFe alloy nanowires, surface-ordered PtFe nanowires demonstrated significantly enhanced durability during long-term operation in both liquid half-cells and fuel cells. In membrane electrode assembly (MEA) testing, their mass activity (MA) exceeded that of non-phase-transformed PtFe nanowires by more than double. After 30,000 cycles of durability testing, they retained 78.6% of their initial value, surpassing the durability target (MA loss < 40%). Their iron loss rate of 41.3% was markedly lower than the 94.1% observed for PtFe NWs without surface ordering.
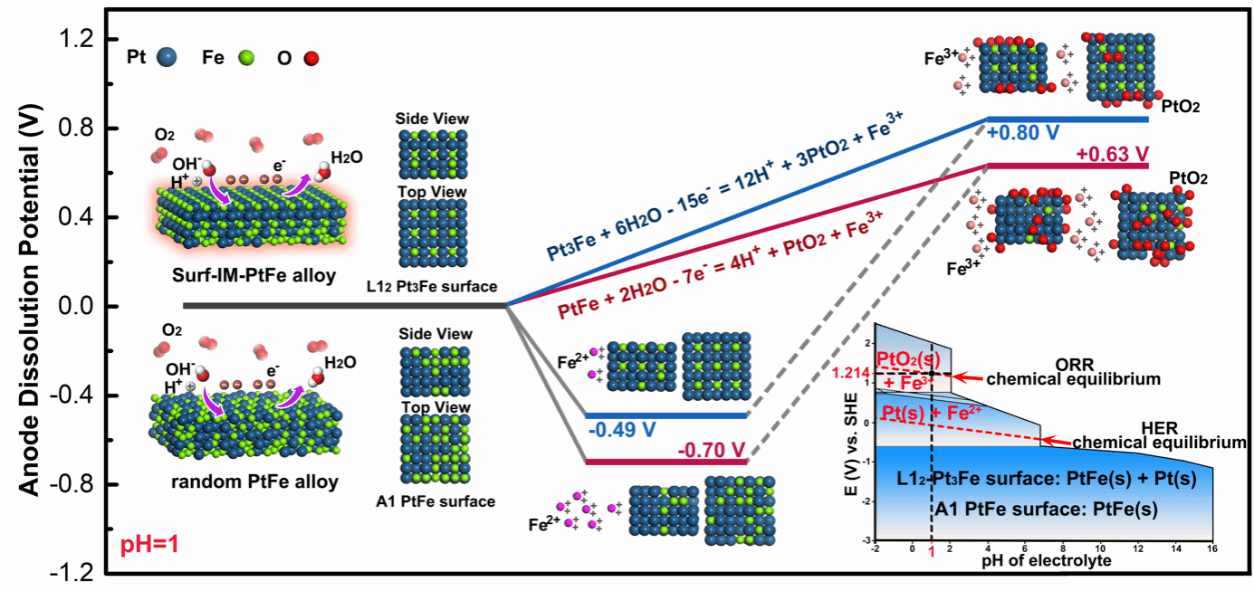
Figure 4. Study of Anodic Dissolution Potential during Couple Corrosion
The decline in activity of Pt-Fe ORR electrocatalysts can be attributed to the inevitable dissolution of Fe under electrocorrosion in acidic electrolytes. However, the corrosion potential of the alloy is challenging to measure experimentally. To better understand the enhanced stability of surface-ordered PtFe nanowire catalysts, we performed density functional theory (DFT) calculations on atomic models of two surface structures: the ordered Li₂₋Pt₃Fe phase and the random Al-PtFe phase. The DFT results further indicate that the formed intermetallic surface exhibits high resistance to galvanic corrosion, significantly suppressing rapid Fe leaching and effectively preventing catalyst degradation.
The design principles developed in this work represent a major breakthrough in addressing scientific and technological challenges at the nanocatalyst level, paving the way for the sustainable application development of highly active fuel cells.
This work was supported by the National Key R&D Programme, the National Natural Science Foundation of China, the Shanghai Outstanding Academic/Technical Leader Programme, the Shanghai Municipal Education Commission Innovation Project, and the Hydrogen Science Centre, the Joint Research Centre for Materials Genome, and the Joint Research Centre for Clean Energy at Shanghai Jiao Tong University.
Full paper link: https://www.science.org/doi/10.1126/sciadv.ado4935
Author: Wu Jianbo's research team
Contributing unit: Centre for Future Materials Innovation






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn