搜索


Research Background:
Wet coating (WC) using N-methylpyrrolidone (NMP) solvent remains the mainstream method for fabricating lithium-ion battery cathode sheets. Despite its maturity, NMP poses toxicity and flammability concerns, while its substantial consumption and energy-intensive drying process during battery fabrication significantly compromise environmental sustainability. Dry electrodes utilising polytetrafluoroethylene (PTFE) binders form fibrous networks under shear stress to anchor active materials and conductive carbon. Nevertheless, the dry-coating preparation of high-energy-density lithium-rich manganese-based cathodes (Li₁.₂Mn₀.₅₄Ni₀.₁₃Co₀.₁₃O₂, LMR), particularly thick electrodes required for rapid charge-discharge conditions in practical applications, remains challenging.
In light of this, the research team led by Dr Sun Hao, Tenured Associate Professor at the Centre for Frontier Science in Transformative Molecules, Shanghai Jiao Tong University, and Resident Scientist at the Zhangjiang Advanced Research Institute, has proposed a thermal-assisted dry coating (TA-DC) method for fabricating high-performance lithium-rich manganese-based cathode sheets. This method employs a low-melting-point additive composed of lithium difluorooxalate borate (LiDFOB) and succinonitrile (SN). Gentle heating melts this additive, promoting uniform distribution of electrode components. Concurrently, the transport pathways formed by LiDFOB and SN facilitate efficient lithium-ion transport within the dry electrode, significantly enhancing the rate performance of lithium-rich manganese cathodes.
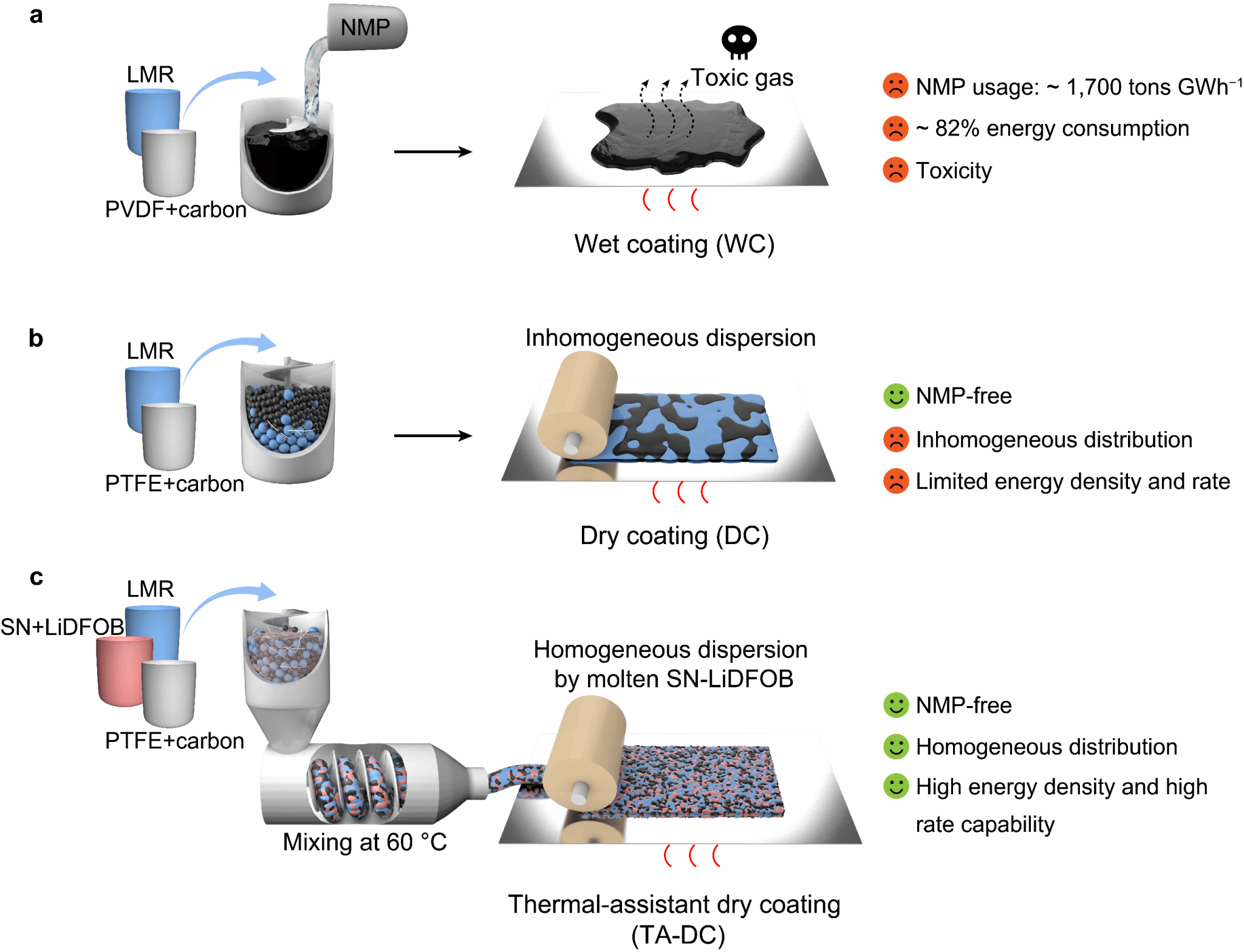
Figure 1. Battery electrode preparation processes and characteristics based on different methods.
The research findings were published in Advanced Materials under the title ‘Thermal-Assisted Dry Coating Electrode Unlocking Sustainable and High-Performance Batteries’. The first authors are Qu Zongtao, Research Assistant at the Centre for Frontier Science in Transformative Molecules; Wang Yan, Assistant Professor; and Zhang Chengxiao, PhD candidate. The corresponding author is Associate Professor Sun Hao, with the primary affiliation being the Centre for Frontier Science in Transformative Molecules at Shanghai Jiao Tong University. This work received substantial support from the National Natural Science Foundation of China, the Special Fund for Basic Research of Central Universities, the Shanghai Natural Science Foundation, the Centre for Frontier Science in Transformative Molecules at Shanghai Jiao Tong University, and the Zhangjiang Advanced Research Institute.
Research Content:
The research team first demonstrated the scalability of electrode fabrication via a thermally assisted dry coating method (Fig. 2a, b). Optical micrographs and scanning electron microscopy images revealed the electrode's excellent structural integrity and uniformity (Fig. 2c-e). Time-of-flight secondary ion mass spectrometry further confirmed the uniform distribution of all components within the electrode (Fig. 2f). Microenvironment conductivity measurements confirmed the stable conductive network within the electrode (Figure 2g). Electrochemical impedance spectroscopy (EIS) was subsequently performed using LMR/LMR symmetric cells to compare lithium-ion transport properties (Figure 2h). Notably, the electrode's ionic resistance was comparable to that of conventional wet-process electrodes, indicating high ionic transport capability. Furthermore, its conductivity reached 2.4 mS cm^(−1) (Figure 2i) – significantly higher than the 0.6 mS cm^(−1) of conventional wet-process electrodes – consistent with microenvironment conductivity measurements (Figure 2g).
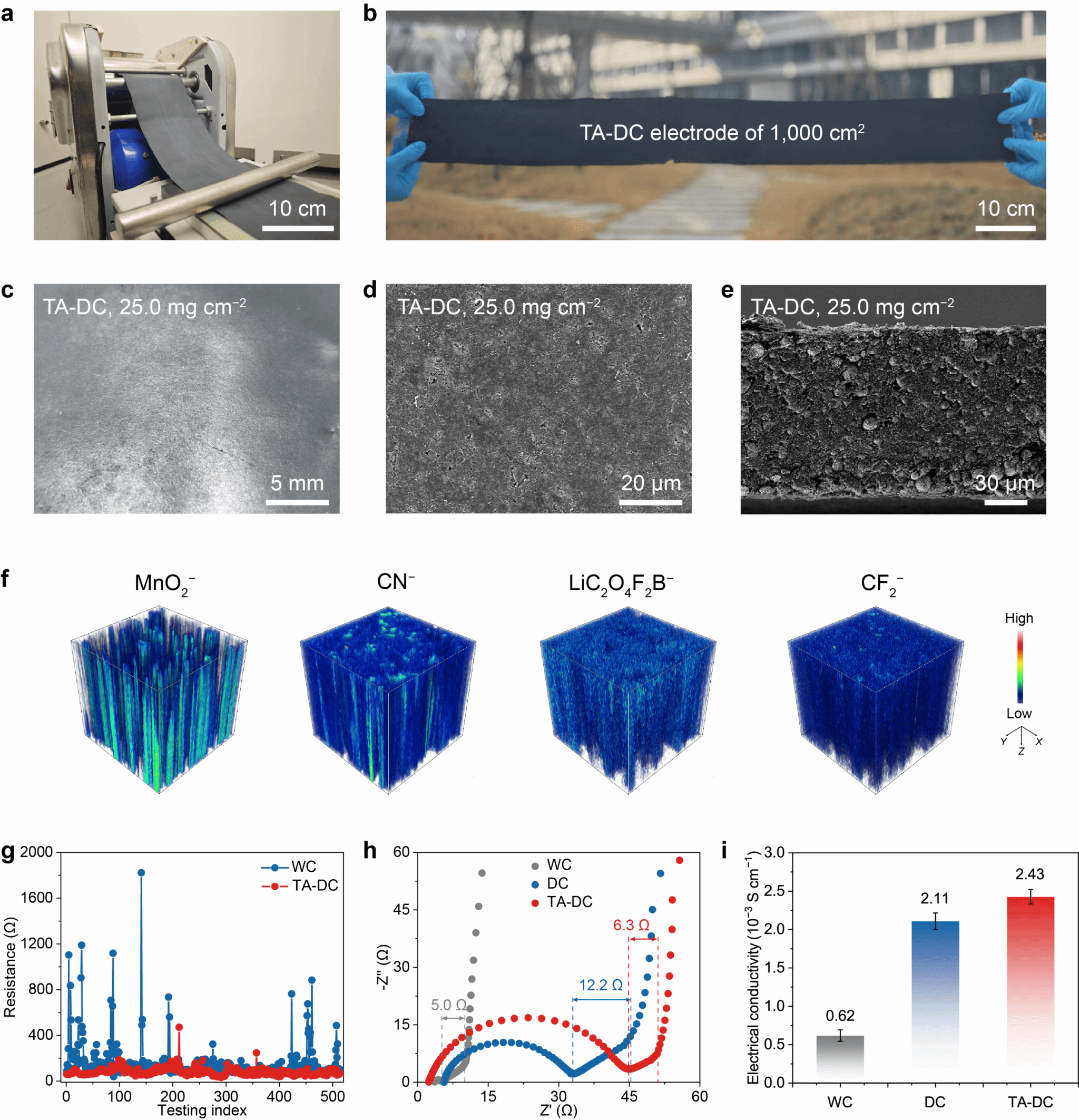
Figure 2. Morphology, distribution, and performance characterisation of thermally assisted dry-processed electrodes.
The research team assembled Li/LMR half-cells using wet-processed (WC), dry-processed (DC), and thermally assisted dry-processed (TA-DC) electrodes with a loading of 11.9 mg cm⁻². The TA-DC electrode exhibited a discharge capacity of 258 mAh g⁻¹ at 0.08 C (Figure 3a). Moreover, the TA-DC electrode exhibited a lower overpotential of 461 mV compared to DC and WC electrodes (Figure 3a). At a rate of 4 C (12 mA cm⁻²), the TA-DC electrode demonstrated a discharge specific capacity of 103 mAh g⁻¹, indicating more efficient ion and electron transport within the electrode (Figure 3b). The maximum energy and power densities, calculated based on LMR mass, reached 1008 Wh kg−1 and 3042 W kg−1 respectively (Figure 3c). Constant potential intermittent titration (GITT) results revealed that the ionic diffusion coefficient of the TA-DC electrode was significantly higher than that of the DC electrode at the same discharge depth (Figure 3d, e). These results demonstrate the enhanced ion and electron transport within the TA-DC electrode. At an LMR loading of 15.7 mg cm⁻², the TA-DC electrode exhibited a capacity retention of 68% after 200 cycles at 0.8 C, whereas the DC electrode retained only 54% capacity (Figure 3f). When the LMR mass loading increased to 29 mg cm⁻², the TA-DC electrode exhibited a capacity retention of 85% after 100 cycles at 0.4 C, compared to 73% for the DC electrode (Figure 3g). Under more demanding conditions with an LMR mass loading of 45.8 mg cm−2 (≈11.0 mAh cm−2), the TA-DC electrode achieved an outstanding discharge capacity of 240 mAh g−1 at 0.08 C (Figure 3h). These results validate the TA-DC electrode's high electrochemical performance even under high capacity loading conditions, demonstrating its potential for practical applications.
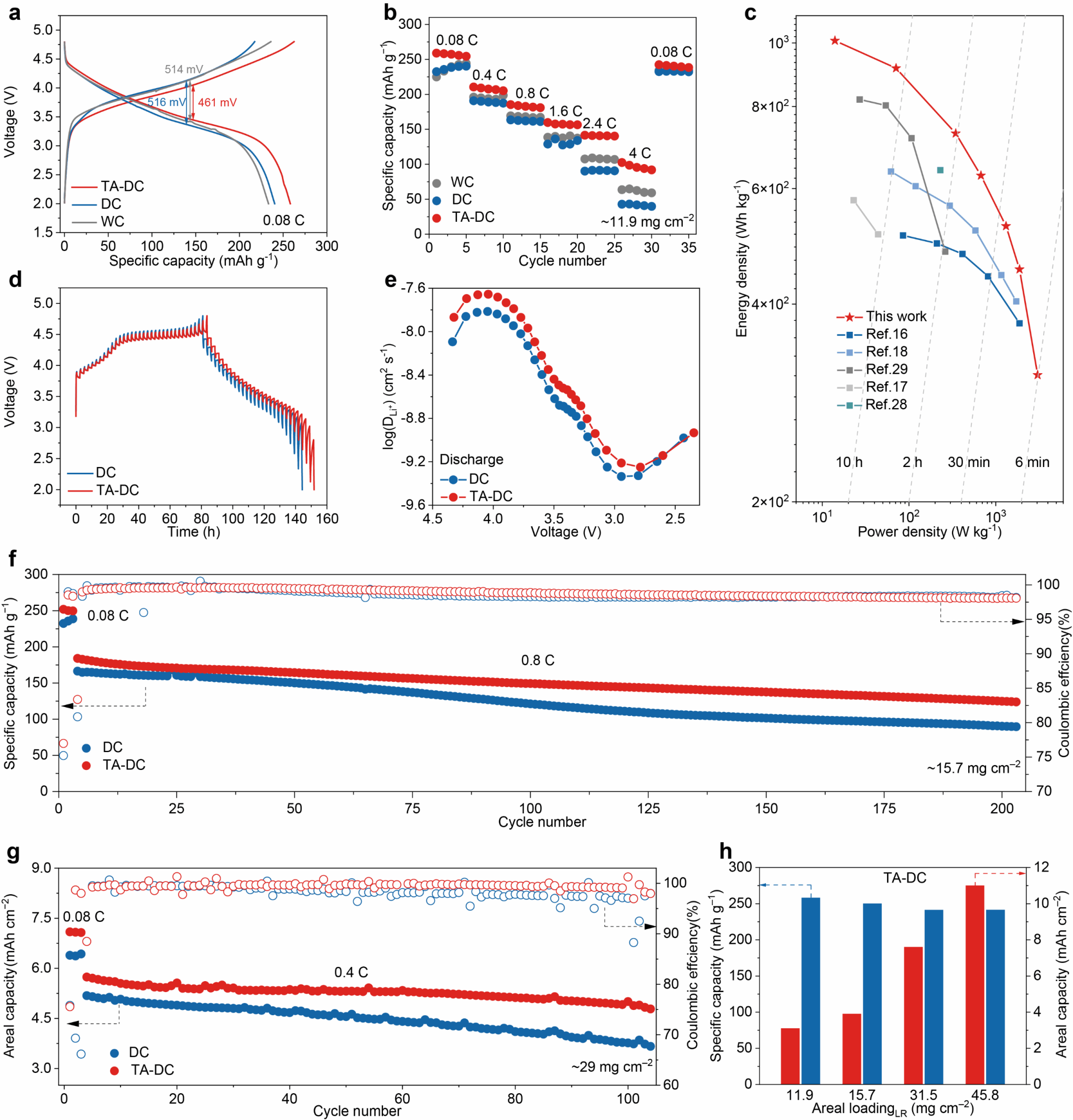
Figure 3. Electrochemical performance study of lithium metal half-cells based on thermally assisted dry-process electrodes.
A Li/LMR full cell was assembled using the TA-DC electrode under a negative/positive capacity ratio (N/P) of 3.1 (Figure 4a). The obtained full cell exhibited a discharge specific capacity of 256 mAh g^(−1) at 0.08 C and demonstrated excellent rate performance at 2.4 C and 4 C (Figure 4b). After 100 cycles at 0.4 C, the capacity retention reached 80%, markedly higher than the 29% observed for DC electrodes (Figure 4c). Based on the total mass of cathode and anode, the Li/LMR full cell achieved maximum energy and power densities of 609 Wh kg−1 and 2183 W kg−1 respectively, demonstrating competitiveness compared to previously reported lithium metal full cells using dry electrodes (Figure 4d). To fully validate the scalability of the TA-DC electrode, the authors fabricated a pouch cell with a discharge capacity of 507 mAh, which maintained 72% capacity after 95 cycles (Figure 4e) and successfully powered a toy electric vehicle (Figure 4f).
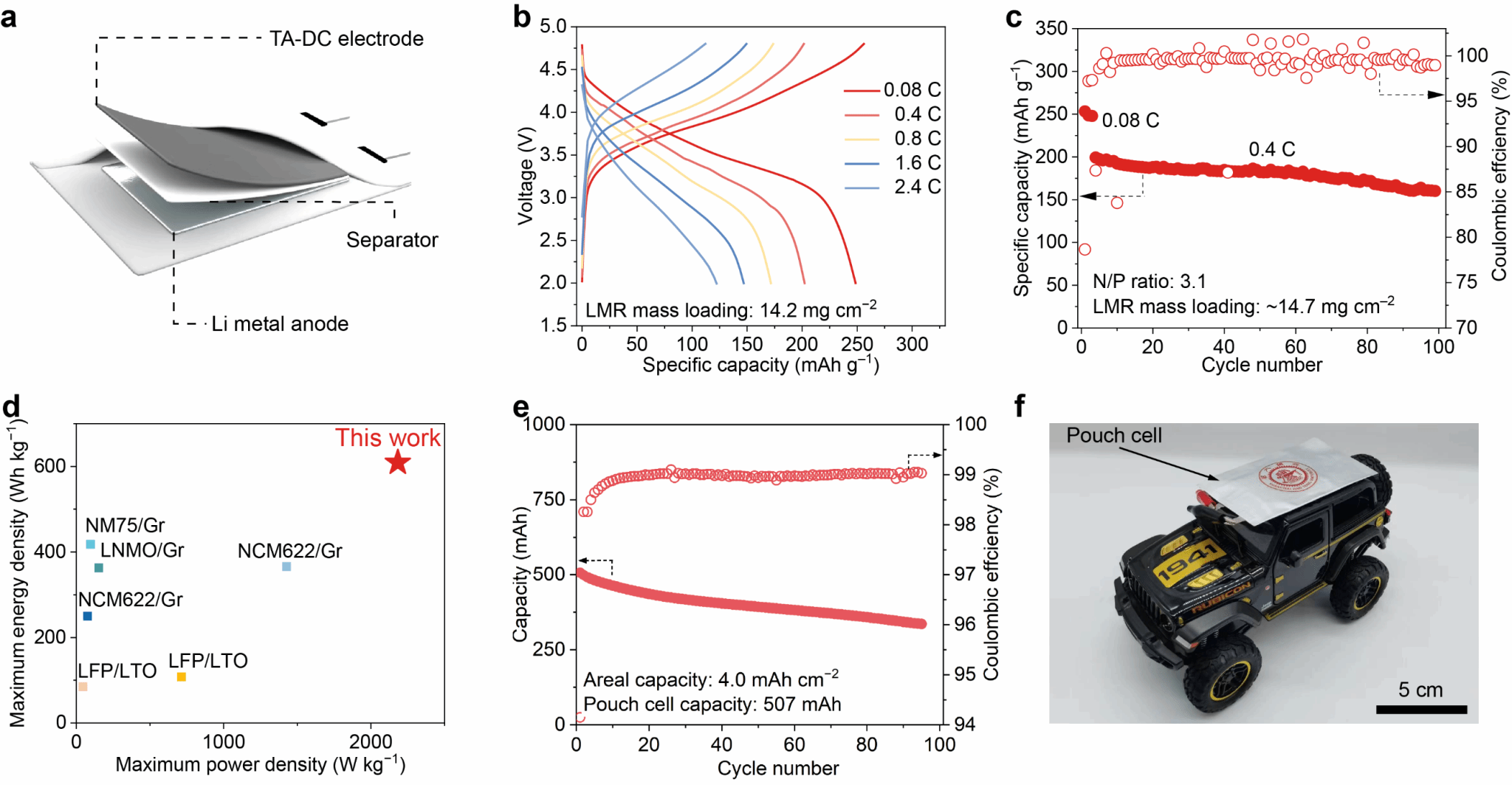
Figure 4. Performance and application demonstration of a lithium metal full cell based on a thermally assisted dry-process electrode.
The research team further employed high-resolution transmission electron microscopy (HR-TEM) and X-ray photoelectron spectroscopy (XPS) to conduct an in-depth analysis of the cathode-electrolyte interface in the TA-DC electrode. Results revealed that, based on the TA-DC method, a uniform interface layer approximately 2 nm thick formed on the surface of cycled LMR particles, with the original crystalline structure of LMR being well preserved during cycling (Figure 5a). In contrast, for the conventional dry-process electrode, a thick and non-uniform interface layer was observed on the surface of cycled LMR particles, accompanied by the emergence of spinel structures (Figure 5b). For the TA-DC electrode after 50 cycles, the F 1s spectrum indicated the formation of a LiF-rich interface layer (Figure 5c). In comparison, the conventional dry-process electrode exhibited lower LiF content but higher levels of LixPOyFz and LixPFy. These findings indicate severe decomposition of LiPF₆ at the electrode/electrolyte interface during battery cycling (Figure 5d). Fourier transform infrared spectroscopy (FT-IR) and density functional theory (DFT) calculations further confirm the existence of coordination between Li⁺ and C≡N, which may promote Li⁺ dissociation from DFOB⁻, thereby enhancing Li⁺ migration (Figures 5f, g). Consequently, the researchers propose that the coexistence of SN and LiDFOB enhances interactions with Li⁺ through abundant functional groups (e.g., C═O, B─F, and C≡N), thereby constructing efficient transport pathways for Li⁺ (Figure 5h).
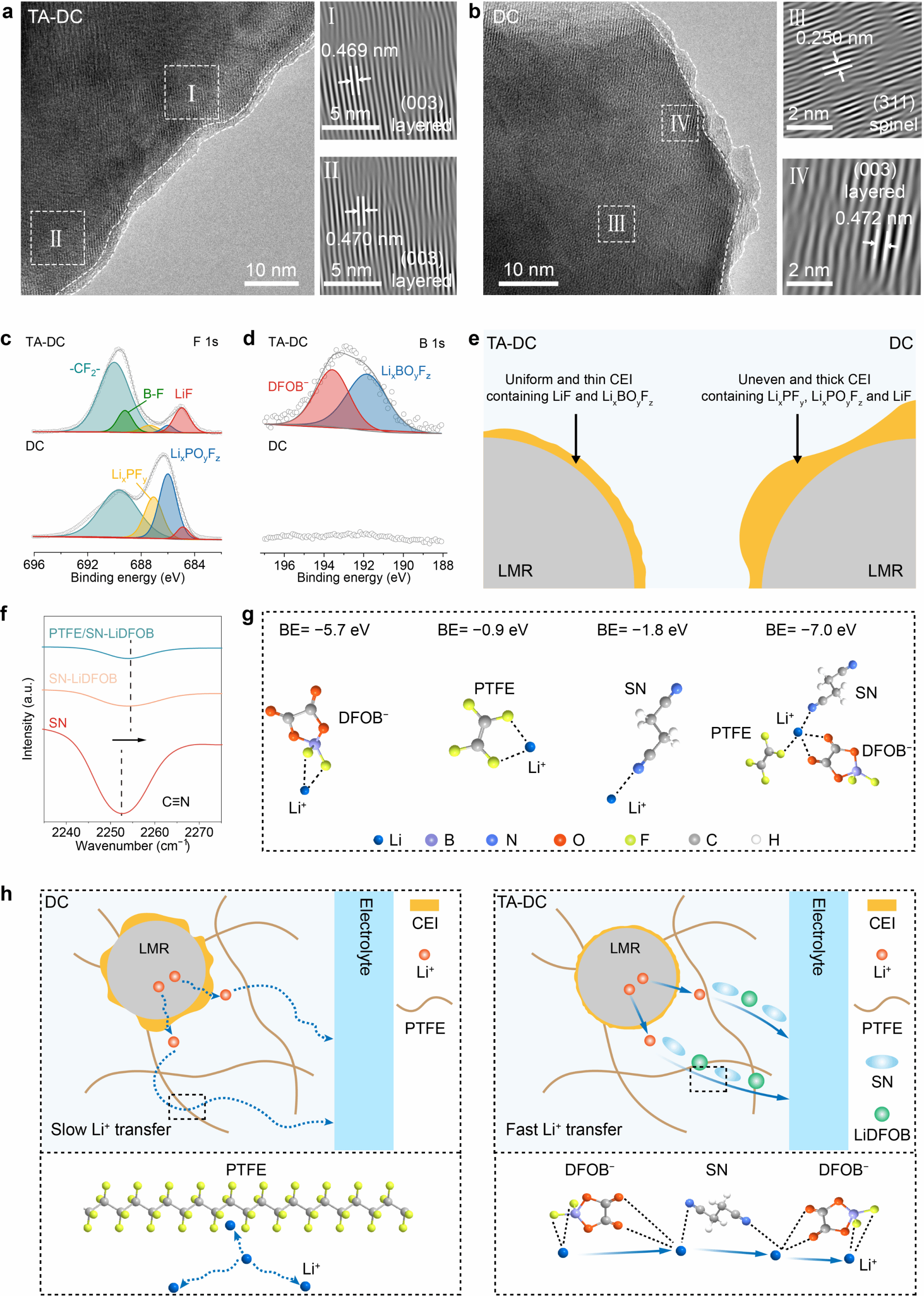
Figure 5. Analysis of the interface and lithium-ion transport properties of the thermally assisted dry electrode.
In summary, this work has developed a thermally assisted dry electrode preparation strategy, achieving solvent-free synthesis of lithium-rich manganese-based cathodes with outstanding energy and power densities. These results provide a novel approach for fabricating dry cathodes for practical applications, offering potential to advance low-cost, sustainable development within the battery industry.
Original link:
https://doi.org/10.1002/adma.202410974
Author: Sun Hao's research group
Contributing institution: Centre for Frontier Science in Transformative Molecules






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn