搜索


Nucleoside and nucleic acid drugs play pivotal roles in medicinal chemistry, chemical biology, and synthetic biology, particularly in developing anticancer and antiviral therapeutics. Chemical modifications enhance pharmacokinetic and pharmacodynamic properties; modifications to sugar rings, bases, or phosphate backbones significantly improve stability and confer diverse functionalities (Figure 1A). C2'-modified nucleosides have garnered significant attention for their ability to enhance nuclease stability and improve binding affinity. However, existing methods for modifying nucleosides, particularly at the C2' position, still face challenges in terms of synthetic efficiency, modularity, and selectivity (Figure 1B). Recently, the research group led by Professor Ming Shang at the Center for Frontier Science in Transformative Molecules, Shanghai Jiao Tong University, and the Zhangjiang Advanced Research Institute, in collaboration with the research groups of Professor Yong Nian at Nanjing University of Chinese Medicine and Professor Weijun Kong at Tongji University, proposed a novel electrochemical nickel-catalyzed strategy. This approach successfully achieved highly stereoselective arylation and alkylation modifications at the C2' position of nucleosides. The researchers also explored the properties of C2'-aryl-modified oligonucleotides (Figure 1C). This work was recently published in Angew. Chem. Int. Ed. under the title “Modular Access to C2'-Aryl/Alkenyl Nucleosides with Electrochemical Stereoselective Cross-Coupling” ( (DOI: 10.1002/anie.202418806). PhD candidate Wang Jiabao is the first author, and Master's candidate Shen Yu is the co-first author. Shanghai Jiao Tong University is the first corresponding institution.
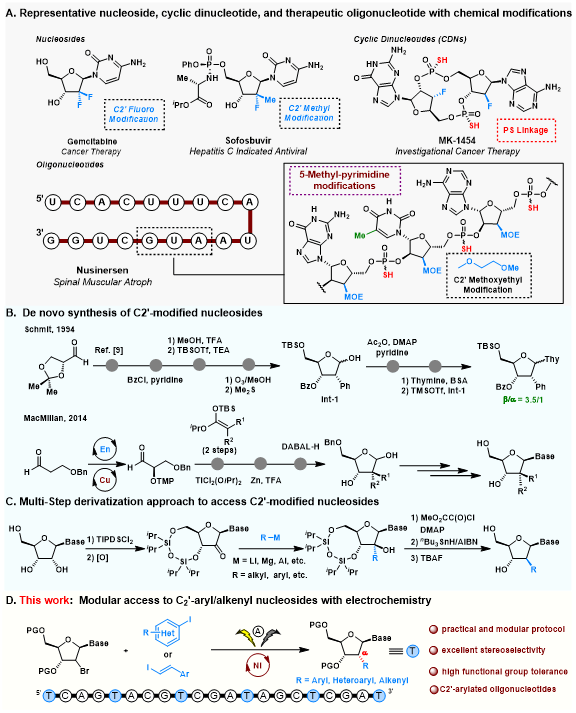
Figure 1. Research Background
Under optimal conditions, the authors investigated the scope of the reaction substrates (Figure 2). Aryl and alkenyl iodides bearing electron-withdrawing, electroneutral, and electron-donating groups, along with various heterocyclic and complex molecules, demonstrated excellent tolerance within this electrochemical system. Uracil, cytosine, and adenine also underwent successful coupling. This demonstrates the reaction's outstanding substrate scope and favourable functional group compatibility, offering broad application prospects for oligonucleotide-based drug development.
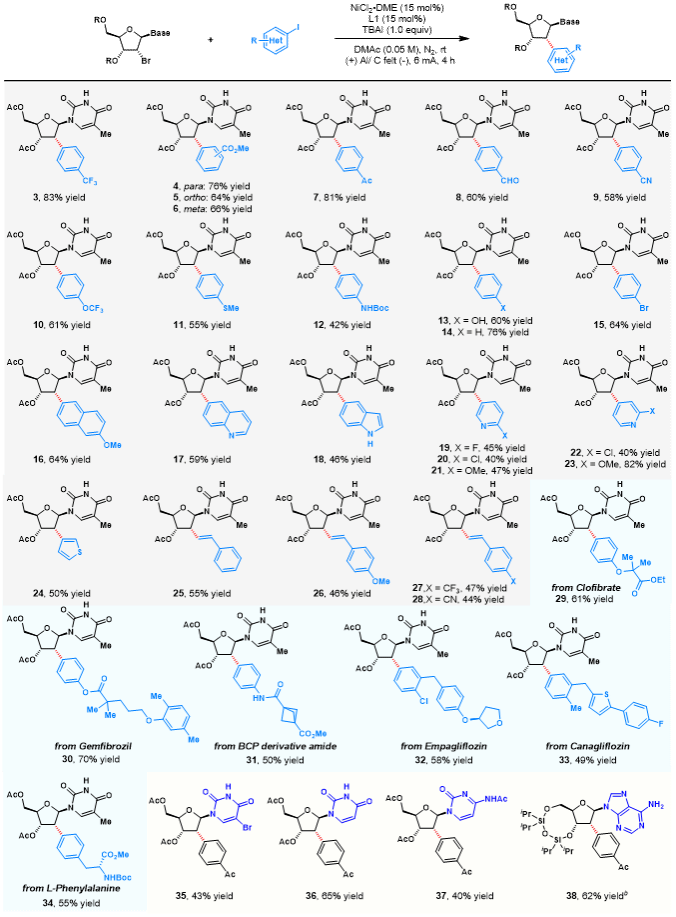
Figure 2. Substrate Expansion
This reaction can also be scaled up, enhancing the practicality of this strategy (Figure 3A). Compared to conventional methods, this approach not only significantly shortens the reaction steps but also substantially improves yields (Figure 3B). To explore the potential applications of C2'-aryl-modified nucleosides in nucleic acid therapeutics, the authors synthesised a series of oligonucleotides bearing thymidine with varying numbers and positions of C2'-4-CF₃-aryl modifications (Figure 3C).
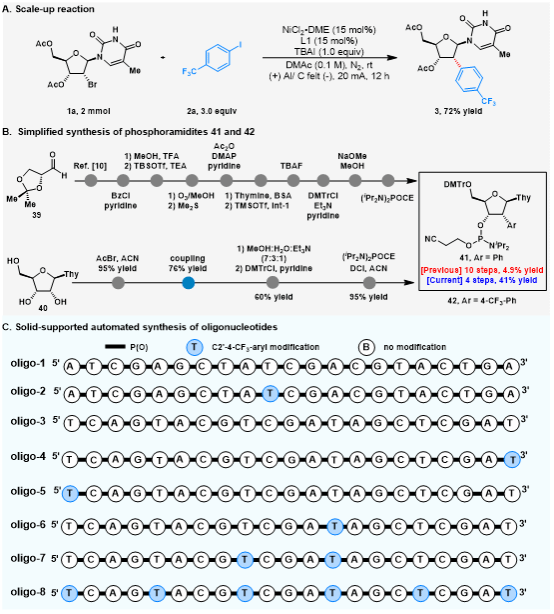
Figure 3. Synthetic Applications
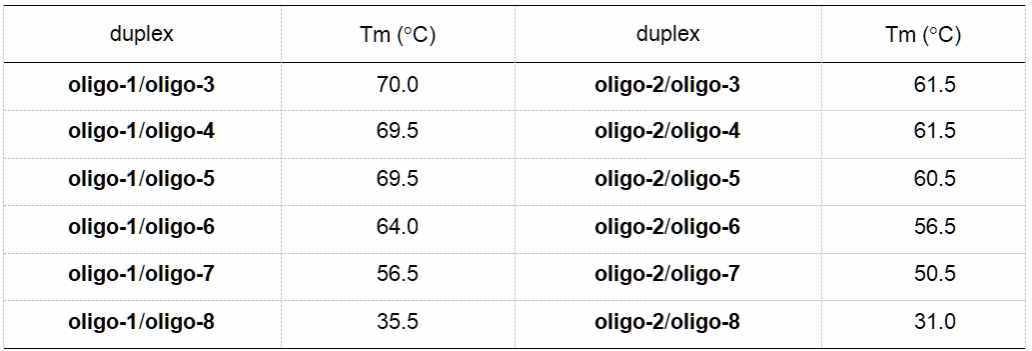
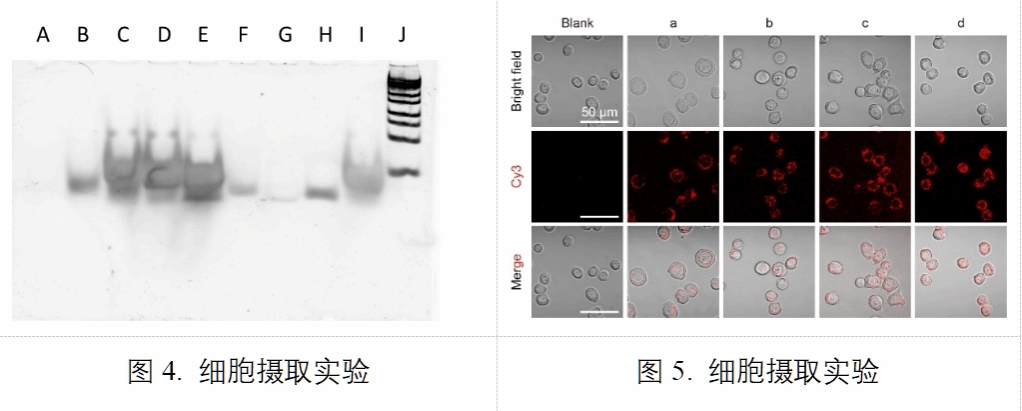
Subsequently, the authors conducted an in-depth investigation into the properties of C2'-aryl-modified oligonucleotides. Firstly, they tested the melting temperatures (Tm) of oligonucleotides with different aryl modifications upon annealing with complementary strands. The results demonstrated that the annealed double-stranded DNA exhibited distinct melting temperatures (Table 1). Secondly, the authors investigated the resistance of 3'-aryl-modified oligonucleotides to 3'-exonuclease activity. Results demonstrated that 3'-aryl-modified oligonucleotides exhibited enhanced exonuclease resistance (Figure 4). Finally, the authors confirmed that oligonucleotides bearing aryl modifications at different positions did not impede cellular uptake processes (Figure 5). These studies highlight the therapeutic potential of C2'-aryl-modified oligonucleotides.
Table 1. Melting temperatures of double strands
In summary, this approach holds significant importance for both synthetic chemistry and biomedical applications. This innovative method introduces a novel perspective to the field of nucleoside-modified synthetic chemistry, streamlining synthetic routes with mild reaction conditions and broad substrate compatibility. It circumvents the stereoselectivity issues associated with Vorbrüggen glycosylation, while its modular design philosophy enables rapid construction of diverse nucleoside derivative libraries. Furthermore, C2'-aryl-modified oligonucleotides exhibit reduced melting temperatures (Tm), enhanced exonuclease resistance, and efficient cellular uptake properties, providing crucial support for future nucleic acid drug development.
Original link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202418806
Author: Shang Ming Team
Contributing Unit: Centre for Frontier Science in Transformative Molecules






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn