搜索



First authors: Liu Zhu, Wang Yongming, Liu Guoquan
Corresponding authors: Liang Zheng, Yan Xuzhou, Yue Xinyang
Corresponding institutions: Centre for Transformative Molecular Frontier Science, Shanghai Jiao Tong University; Zhangjiang Advanced Research Institute, Shanghai Jiao Tong University
The teams of Zheng Liang and Xu Zhou Yan, resident scientists at the Centre for Transformative Molecular Frontier Science and Zhangjiang Advanced Research Institute of Shanghai Jiao Tong University, collaborated to successfully synthesise a mechanical interlocking network (MIN)-based binder through the self-assembly of supramolecular units combined with UV-induced thiol-ene click chemistry. This binder was subsequently applied to pure silicon anodes in lithium-ion batteries (LIBs). This MIN-based binder comprises three components: a [an]daisy-chain-containing MIN (denoted as DCMIN), a thiol-functionalised 2-ureidopyrimidinone (UPy-SH) unit, and polyacrylic acid (PAA). The resulting DCMIN@PAA binder containing 5 wt% DCMIN exhibits more balanced mechanical properties, with a fracture stress of 16.5 MPa, elongation at break of 340%, and toughness of 32.1 MJ m–3. Effective energy dissipation behaviour under synergistic mechanical bond motion within the damage-free binder was demonstrated via strain-stress curves, atomic force microscopy, and molecular dynamics (MD) simulations. Results indicate that pure silicon anodes using the DCMIN@PAA damage-free binder significantly outperform anodes with other covalent binders in terms of capacity retention and long-term cycling performance (exceeding 1050 cycles at 1C). Furthermore, DCMIN@PAA粘结剂在高负载的微米级Si@石墨负极和LiNi0.8Co0.1Mn0.1O2软包电池中优势明显. Notably, the supramolecular network-based DCMIN@PAA binder rapidly degrades in alkaline aqueous solutions, enabling swift release of active material from electrode sheets. This facilitates more convenient and environmentally sound disposal of spent active materials. The design philosophy of this sustainable auxiliary material and its “rapid release” binder functionality contribute to the recycling of end-of-life batteries. The related research findings, titled ‘Durable and Damageless Supramolecular Binder for Fast, Stable, and Sustainable Si-based Anodes’, were published in the Journal of the American Chemical Society.
【Research Background】
Silicon (Si) is widely regarded as a preferred alternative to conventional graphite anodes in high-energy-density lithium-ion batteries (LIBs) due to its high specific capacity (3592 mA h g−1), low cost, and favourable operating potential (approximately 0.4 V vs Li/Li+). However, silicon undergoes up to 300% volume expansion during full lithiation, inevitably hindering electron transport and causing repeated formation of the solid electrolyte interphase (SEI), thereby shortening LIB lifespan. Currently, reducing silicon particle size to the nanoscale or introducing carbon matrices can partially mitigate the volume effect. Nevertheless, stresses/energies arising from silicon anode expansion/contraction are absorbed by the cross-linked polymer binder within the anode, creating damage points within the binder network. Once accumulated damage reaches a critical threshold for mechanical fatigue, both the binder structure and the anode itself may undergo abrupt collapse. Therefore, dispersing energy to prevent damage accumulation within the binder network is crucial for enhancing silicon-based anode performance. Although existing binder design strategies combining hard and soft chains offer good elasticity and tensile strength, achieving some success, these approaches remain insufficient in completely suppressing the generation of damage sites and subsequent mechanical fatigue of the binder. Furthermore, conventional binder designs have placed excessive emphasis on high adhesion properties. This characteristic, however, becomes an obstacle during post-failure battery recycling processes in practical applications, particularly in battery dismantling, sorting, and active material recovery stages. Consequently, there is an urgent need for further optimisation and refinement of the design philosophy for battery electrode binders.
【Research Content】
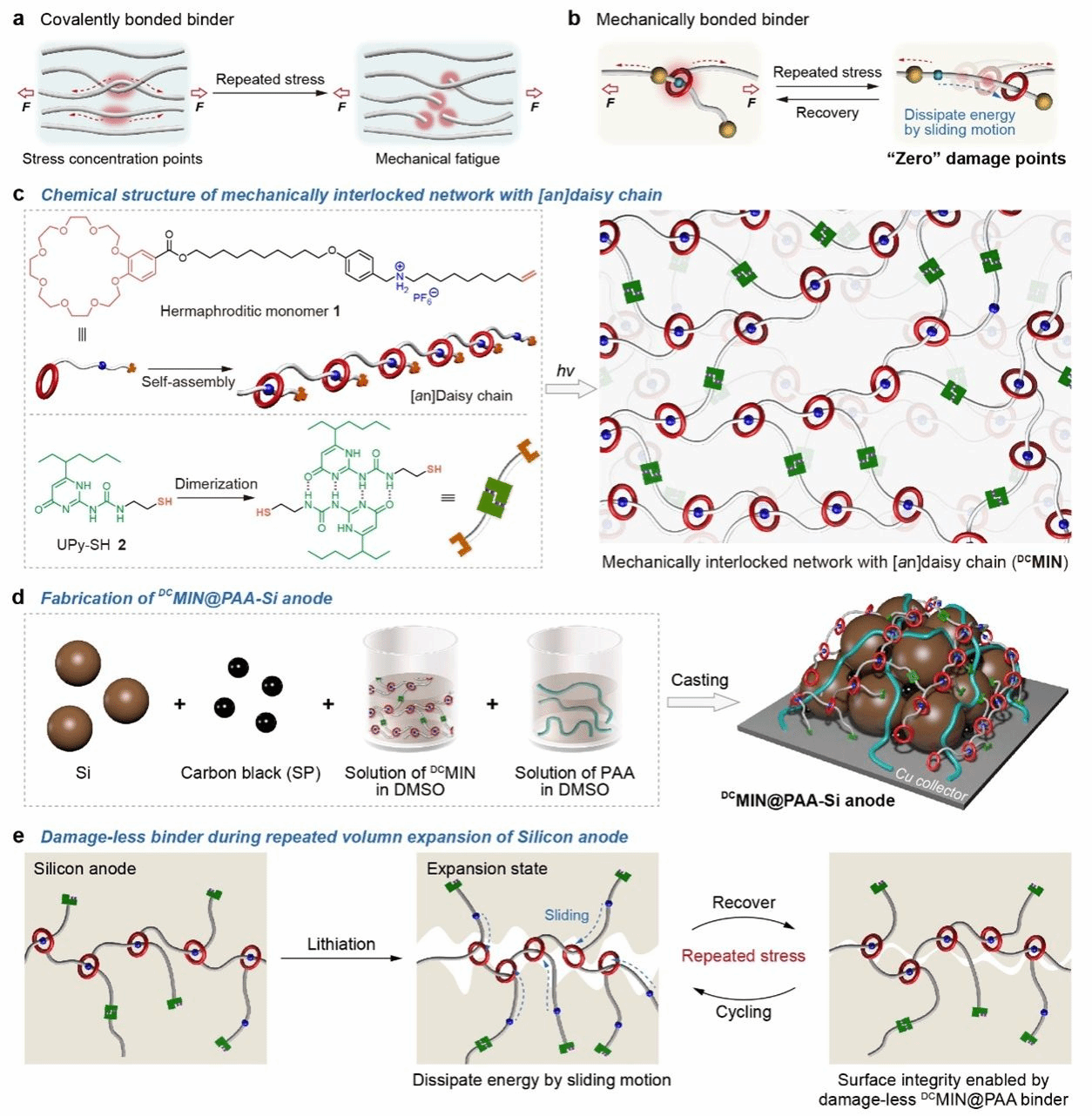
Figure 1: Design, Preparation and Mechanism of DCMINs@PAA Binder
Figure 1 highlights that within silicon-based anodes, employing a binder featuring a mechanically interlocked network structure can effectively dissipate stresses induced by silicon particle volume changes. This reduces the formation and accumulation of damage points, thereby enhancing anode stability and battery cycle life. Specifically, Figure 1(a) illustrates the formation of damage points on a conventional covalent bond network (CBN) binder during volume changes in silicon-based anode materials. These damage points accumulate with alternating stress cycles, ultimately leading to the collapse of both the binder network and the anode structure. Figure 1(b) illustrates the Mechanically Interlocked Networks (MINs) structure, which achieves ‘zero’ damage points by rapidly dissipating energy through the motion of mechanical bonds, thereby reducing damage accumulation. Figure 1(c) depicts, in cartoon form, the process of forming DCMI (Daisy Chains MINs) via supramolecular polymerisation, followed by reaction with UPy-SH (a thiol compound containing 2-ureido-4-pyrimidinone) through a sulphur-ene click reaction to form the mechanically interlocked network. Figure 1(d) depicts the fabrication process for silicon anodes using DCMIN@PAA binder. Figure 1(e) highlights the mechanism of DCMIN@PAA in stabilising silicon anodes, emphasising the critical role of energy dissipation in enhancing silicon anode performance.
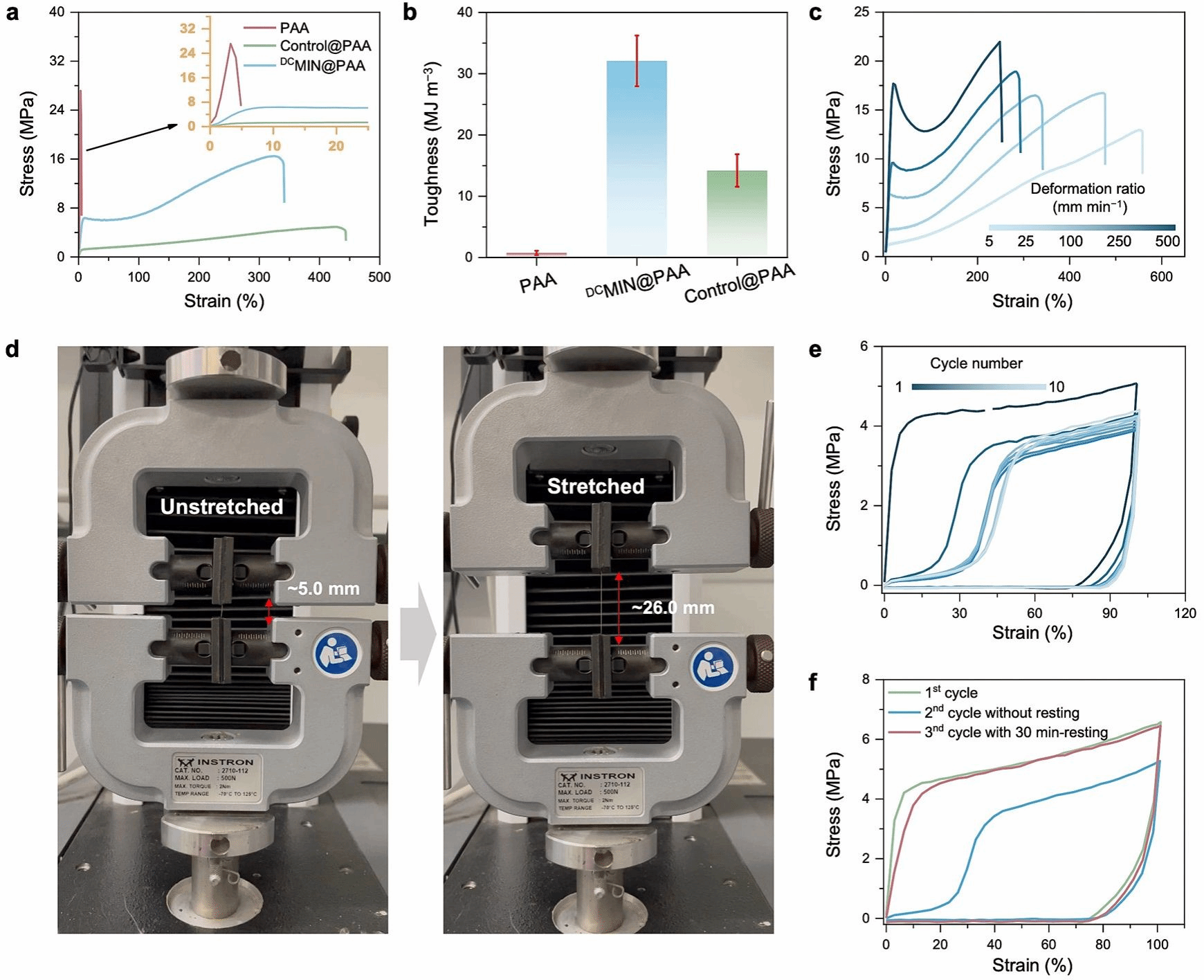
Figure 2: Mechanical property analysis of DCMIN@PAA, Control@PAA and PAA.
Furthermore, Figure 2 demonstrates through experimentation and simulation the mechanical properties of the DCMIN@PAA binder, particularly its energy dissipation capacity and structural stability under varying conditions. These characteristics are crucial for enhancing the performance of silicon-based anodes. Comparing stress-strain curves (Figure 2(a)) and toughness values (Figure 2(b)), DCMIN@PAA exhibits superior mechanical properties compared to PAA and Control@PAA, including higher fracture stress, greater elongation, and enhanced toughness. Figure 2(c) illustrates the effect of different tensile rates on DCMIN@PAA, demonstrating its ability to maintain mechanical properties across varying speeds – a critical factor for practical application adaptability. Figures 2(e-f) demonstrate the energy dissipation capability of DCMIN@PAA through load-unload cycles and cyclic tensile curves, indicating its ability to absorb and dissipate energy during cyclic loading, thereby reducing damage accumulation. Molecular dynamics (MD) simulations (Figures 2(g–i)) reveal the dynamic behaviour of DCMIN@PAA at the molecular level, including hydrogen bond formation, chain motion, and the dissociation and reconstruction of DCMIN host–guest recognition. These processes underpin the macroscopic manifestation of DCMIN@PAA's outstanding mechanical properties and energy dissipation capabilities.
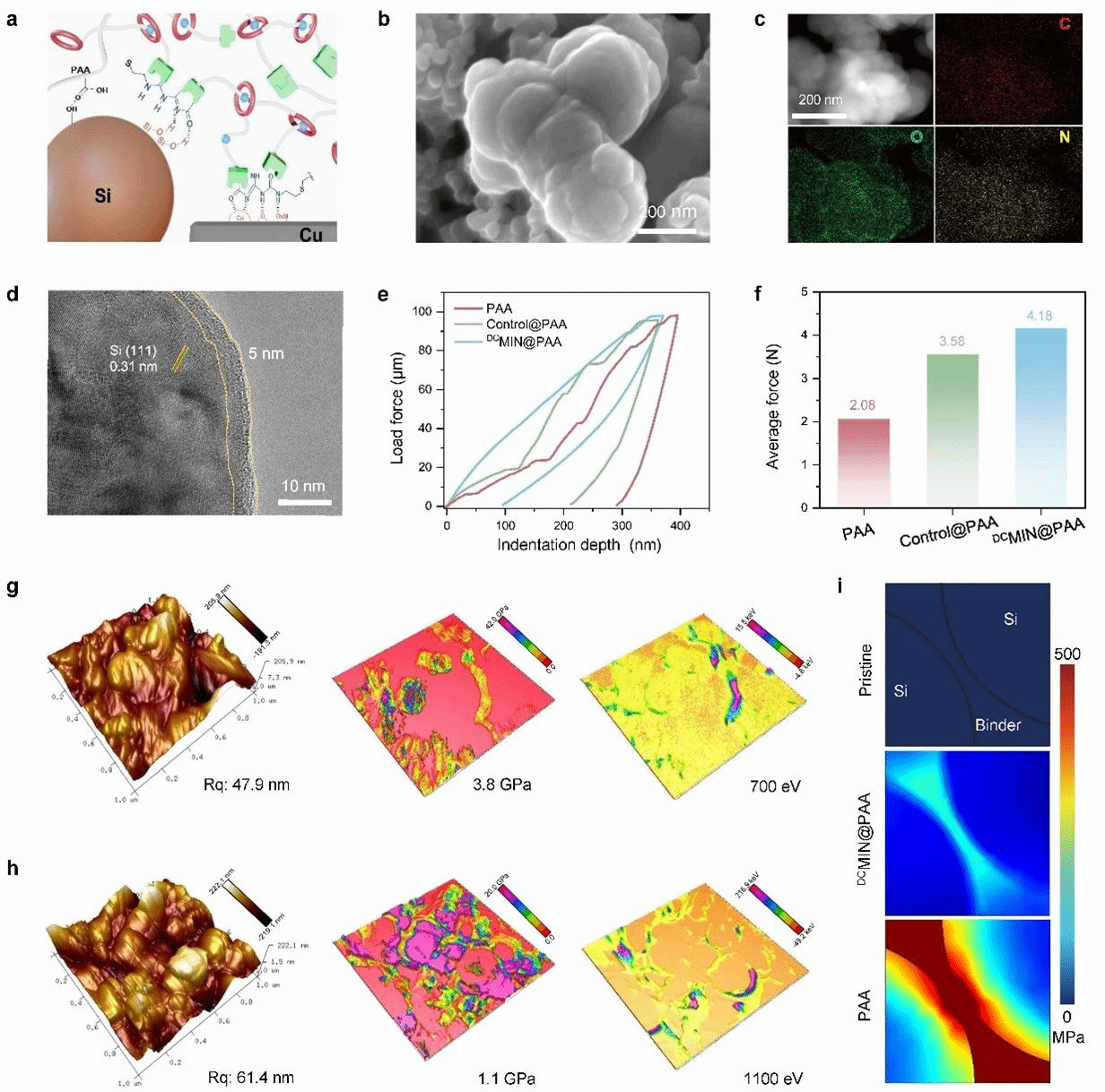
Figure 3: Characterisation of DCMIN@PAA-Si.
Figure 3 further elucidates the application efficacy of the DCMIN@PAA binder within silicon-based anodes, encompassing its enhanced adhesion, improved mechanical properties, and structural stability. Benefiting from the synergistic effects of multiple hydrogen bonds and host-guest recognition, Figure 3(a) schematically illustrates the interaction mechanism between silicon (Si) particles, the DCMIN@PAA binder, and the copper current collector. Figures 3(b–c) present SEM images of DCMIN@PAA-Si, revealing uniform binder distribution across silicon particles. Figure 3(d) further confirms the thin, even coating of DCMIN@PAA binder on silicon particles. Nanoindentation testing of anodes prepared with different binders demonstrated smooth loading curves and the highest elastic recovery for DCMIN@PAA. The 180° peel test demonstrated enhanced adhesion of the DCMIN@PAA binder, which is advantageous for preparing high-loading silicon anode materials. Further characterisation of DCMIN@PAA-Si using atomic force microscopy (Figure 3(g-h)) revealed that energy induced by Si deformation could be dissipated promptly through the cooperative motion of [an]DCs within the DCMIN@PAA binder. Finite element simulations (Figure 3i) provide further evidence. Upon full lithiation (Li₂₂Si₅), the PAA system exhibits severe and non-uniform stress concentration on the Si surface, whereas only minor stress concentration forms within the DCMIN@PAA-Si particles. In summary, the DCMIN@PAA-Si anode exhibits outstanding mechanical stability and rapid energy dissipation capabilities, contributing to the stable cycling of high-energy-density lithium-ion batteries by overcoming energy/damage accumulation and mechanical fatigue within the binder network.
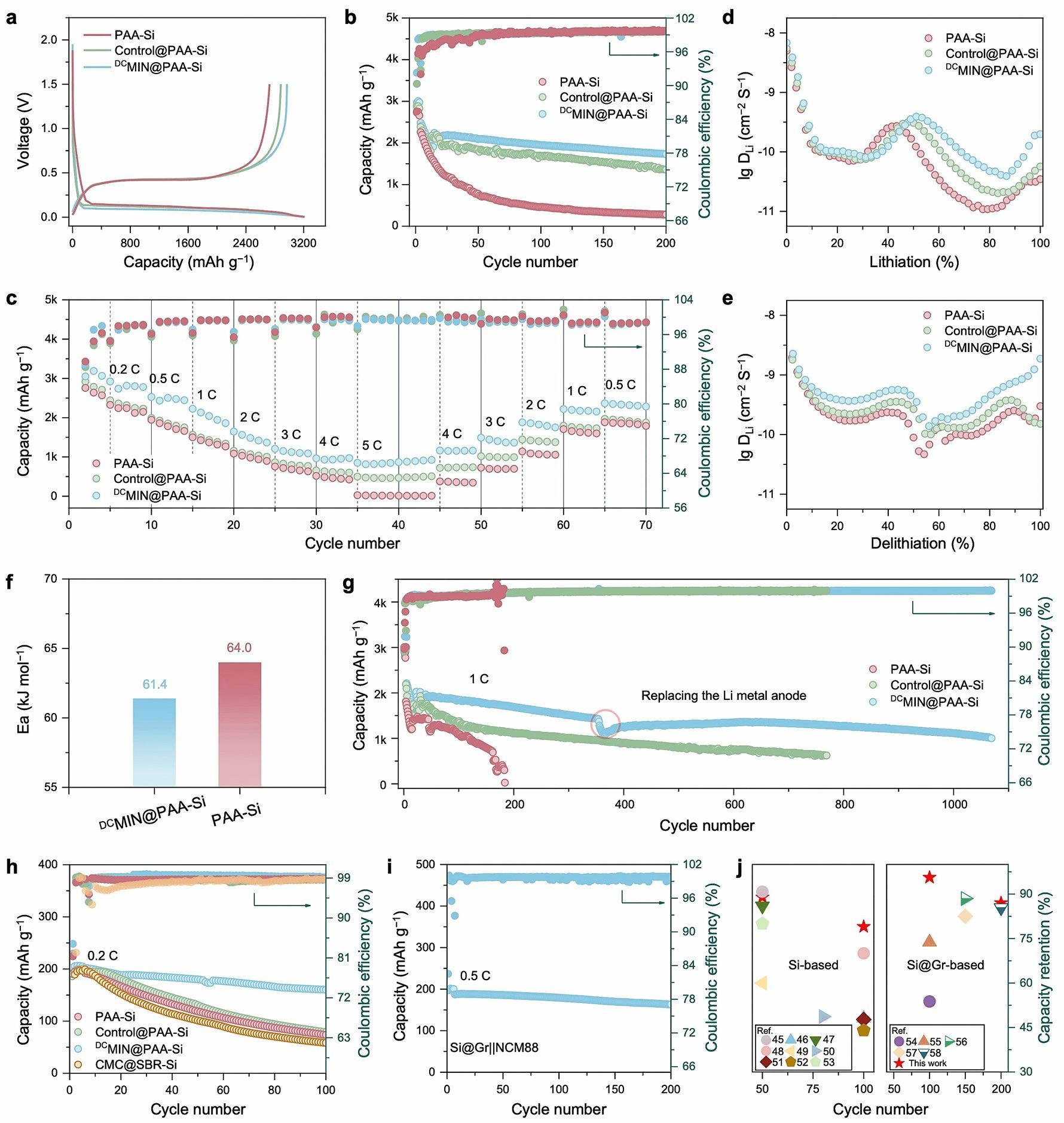
Figure 4: Electrochemical and Battery Performance of DCMIN@PAA-Si
Figure 4 further demonstrates the application performance of the DCMIN@PAA binder in silicon anode lithium-ion batteries, including higher reversible capacity and coulombic efficiency during initial cycling, stable cycling performance and structural integrity, as well as superior rate performance compared to PAA-Si and Control@PAA-Si anodes. Through synergistic non-covalent interactions and host-guest recognition, the substantial volumetric expansion energy of silicon anodes is rapidly released. DCMIN@PAA-Si exhibits high structural integrity with stable SEI formation throughout cycling. Consequently, even at high silicon loading (2.2 mg cm⁻²), the battery performance surpasses the control group under the synergistic effect of the DCMIN@PAA binder. Owing to the stable SEI on DCMIN@PAA-Si and its reduced solvent-derived organic content, the activation energy (Ea) for lithium ion penetration through the SEI is merely 61.4 kJ mol–1, lower than that of PAA (64.0 kJ mol–1). Consequently, DCMIN@PAA-Si exhibits superior reaction kinetics compared to PAA-Si. Beyond the SEI, interactions between lithium ions and DCMIN@PAA may also influence the rate performance of silicon anodes. Our prior research revealed that the ether group of B24C8 within DCs can coordinate with lithium ions, enabling synergistic movement that ‘trains’ lithium ions through DCMIN to facilitate transport. This finding was validated via constant current intermittent titration (GITT) and variable sweep rate cyclic voltammetry (CV). Owing to its stable structure, rapid energy dissipation, and enhanced lithium-ion kinetics, the DCMIN@PAA binder delivers exceptional cycling capability for pure silicon anodes under high-rate cycling. It achieves a reversible capacity of 1008.4 mAh g–1 after over 1000 cycles while maintaining low polarisation, demonstrating significant advantages over PAA and other relevant binders. The practicality of DCMIN@PAA was further validated through button cells and pouch cells. Testing a custom-made 100 mAh LiNi₀.₈Co₀.₁Mn₀.₁O₂ (NCM811) || Si pouch cell revealed that after 100 cycles at 0.5 C, DCMIN@ PAA-based cells exhibited a capacity retention rate of 80.4%. Furthermore, employing DCMIN@PAA as the anode binder facilitates easier recycling. Failed anodes degrade rapidly upon simple immersion in an alkaline aqueous solution, enabling swift release and separation of active material from the current collector, thereby enhancing battery sustainability.
【Conclusion and Outlook】
This study designed an ultra-durable DCMIN@PAA binder (incorporating [an]daisy chains within a mechanically interlocked network and polyacrylic acid). Within a single system, it achieves strong interfacial bonding, efficient energy dissipation, and rapid release/separation design. Through the motion of the mechanically interlocked network and partial dissociation of hydrogen bonds, the DCMIN@PAA binder rapidly dissipates deformation energy induced by silicon volume expansion. This maintains binder network stability, reduces the formation and accumulation of damage sites, and further limits mechanical fatigue of the anode. With a reversible capacity of 1542.8 mA h g−1 and high capacity retention after 300 cycles, the pure silicon anode utilising DCMIN@PAA binder demonstrated exceptional performance. Even at high rates, DCMIN@PAA-Si exhibited remarkable long-term cycling stability exceeding 1000 cycles. Moreover, pure silicon anodes utilising the DCMIN@PAA binder within pouch cells at 100 mA h also demonstrated competitive cycling capability. Notably, the DCMIN@PAA binder rapidly degrades in alkaline aqueous solutions, exhibiting rapid release and separation properties that facilitate more convenient and environmentally sound recycling of these anodes. This research provides a novel approach for designing high-energy-density lithium-ion battery anodes susceptible to volume expansion. The paper emphasises the critical role of energy dissipation in binder design and offers a viable development pathway for stabilising silicon-based anodes against stress accumulation induced by volume changes.
【Bibliographic Information】
Zhu Liu#, Wang Yongming#, Liu Guoquan#, Yue Xinyang*, Shi Zhangqin, Tan Yihong, Zhao Jun, Lei Yu, Yan Xuzhou*, Liang Zheng*, A Durable and Non-Destructive Supramolecular Adhesive for Rapid, Stable and Sustainable Silicon-Based Anodes. Journal of the American Chemical Society, 2024, 10.1021/jacs.4c11217
【Original Link】
https://doi.org/10.1021/jacs.4c11217
Contributing Unit:






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn