搜索


C-glycosides are typically more stable than traditional O-glycosides and N-glycosides, exhibiting superior biological stability in vivo and resistance to degradation by hydrolases. This makes the study and synthesis of C-glycosides highly significant in medicinal chemistry and natural product chemistry (Figure 1a). As key intermediates for synthesizing diverse C-glycosides and natural products (see Figure 1b), developing concise and efficient methods for obtaining C-1 glycene is essential. The Suzuki-Miyaura cross-coupling reaction involving C-1 glycene boron reagents has garnered extensive attention and application due to its mild conditions, high efficiency, and broad applicability; numerous natural C-glycoside molecules have been successfully synthesized (Figure 1c). However, the large-scale preparation of sugar-boron compounds remains a significant challenge. Due to the electron-rich unsaturated enol ether structure of C-1 sugar-boron compounds, they exhibit poor stability and are difficult to purify, often requiring specialized experimental operations and handling. This characteristic has prevented the synthesis of polysaccharide vinylboranes to date, and no reports exist on the post-synthesis glycosylation of complex molecules using sugar vinylborane reagents under biocompatible conditions. Therefore, developing sugar vinylborane reagents that combine excellent stability with outstanding reactivity to achieve highly modular C-1 sugar vinyl synthesis under mild conditions holds significant scientific importance and application potential.
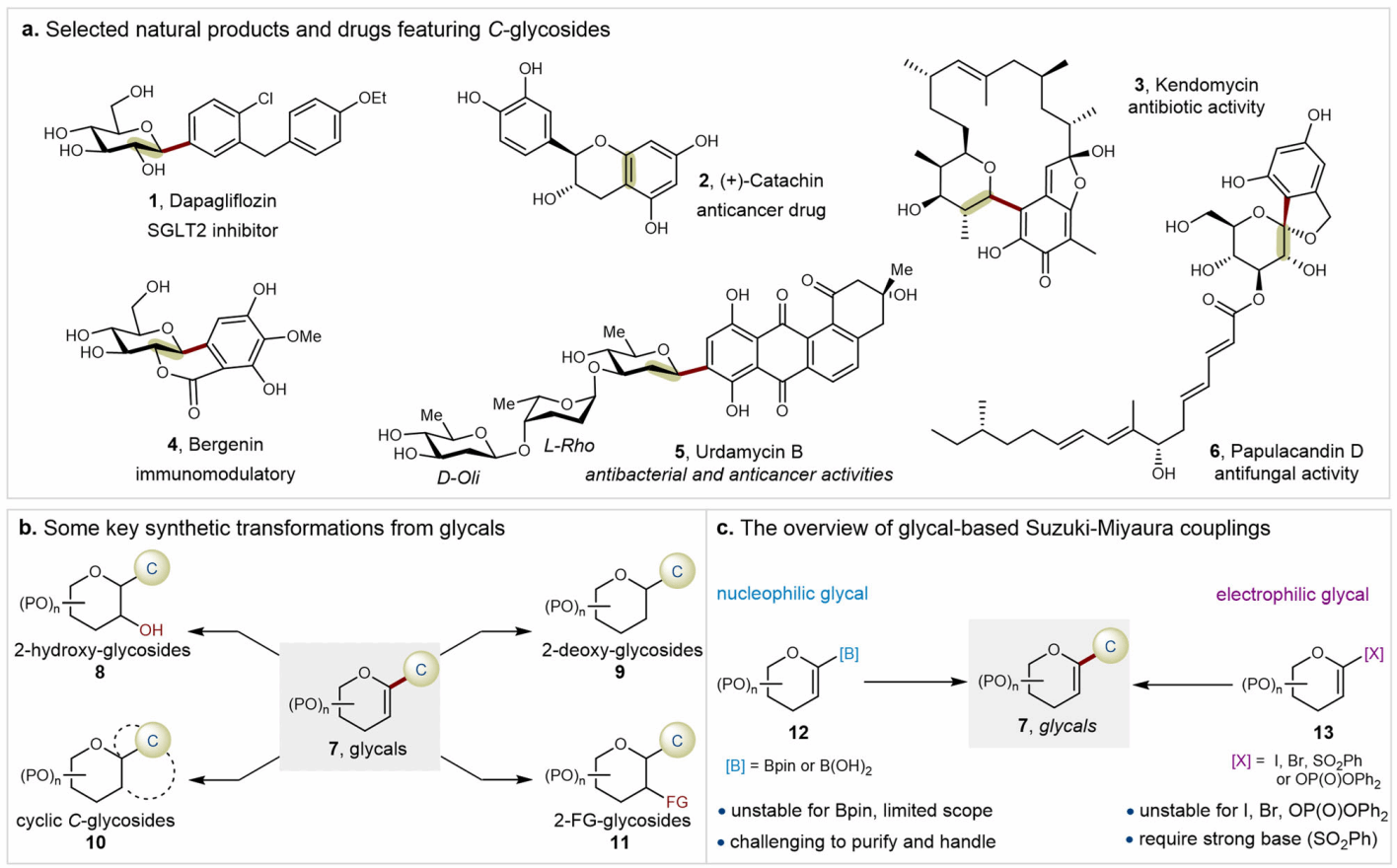
Figure 1. Research Background
To address these challenges, the research group led by Dr. Feng Zhu—a resident scientist at the Center for Transformative Molecular Science at Shanghai Jiao Tong University and the Zhangjiang Institute for Advanced Studies—developed two classes of stable and reactive sugar-alkene boron reagents. These reagents utilize 1,1,2, 2-tetraethylglycol and (+)-pinacol diol as boronic acid protecting groups, successfully achieving a highly modular palladium-catalyzed sugar-alkene Suzuki-Miyaura cross-coupling reaction. This method robustly constructs Csp2-Csp, Csp2-Csp2, and Csp2-Csp3 bonds under mild conditions with good to excellent yields (Figure 2).
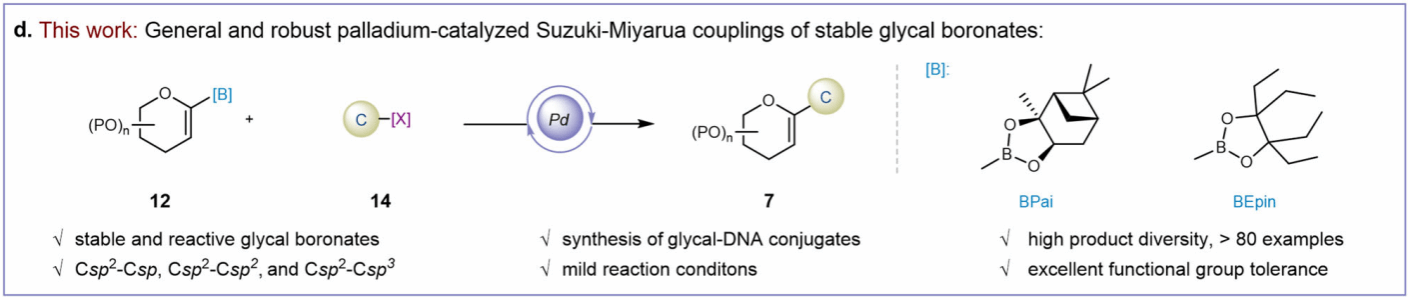
Figure 2. Research Methodology
First, under optimized conditions, the authors conducted a comprehensive investigation of the range of electrophiles in this reaction (Figure 3). Results demonstrated that the reaction is compatible with diverse functional groups, including electron-withdrawing, electron-donating groups or heterocycles, as well as reactive functional groups (e.g., hydroxyl, amino, carboxyl). Simultaneously, the authors tackled more challenging oligopeptide substrates. Encouragingly, these substrates also yielded the desired products under mild reaction conditions. To validate the method's universality, the authors performed post-synthesis glycosylation of drug molecules and natural products, achieving moderate to good yields of the target products. Subsequently, the authors extended this approach to glycan modification of biomacromolecules, developing the first DNA-compatible Suzuki-Miyaura-type glyco-crosscoupling reaction. This enabled the successful synthesis of diverse novel glyco-DNA conjugates. Furthermore, a one-pot strategy was devised to achieve the tandem C-H boronation and crosscoupling of glyco-molecules, enabling one-step C-glycosylation of various glyco-molecules.
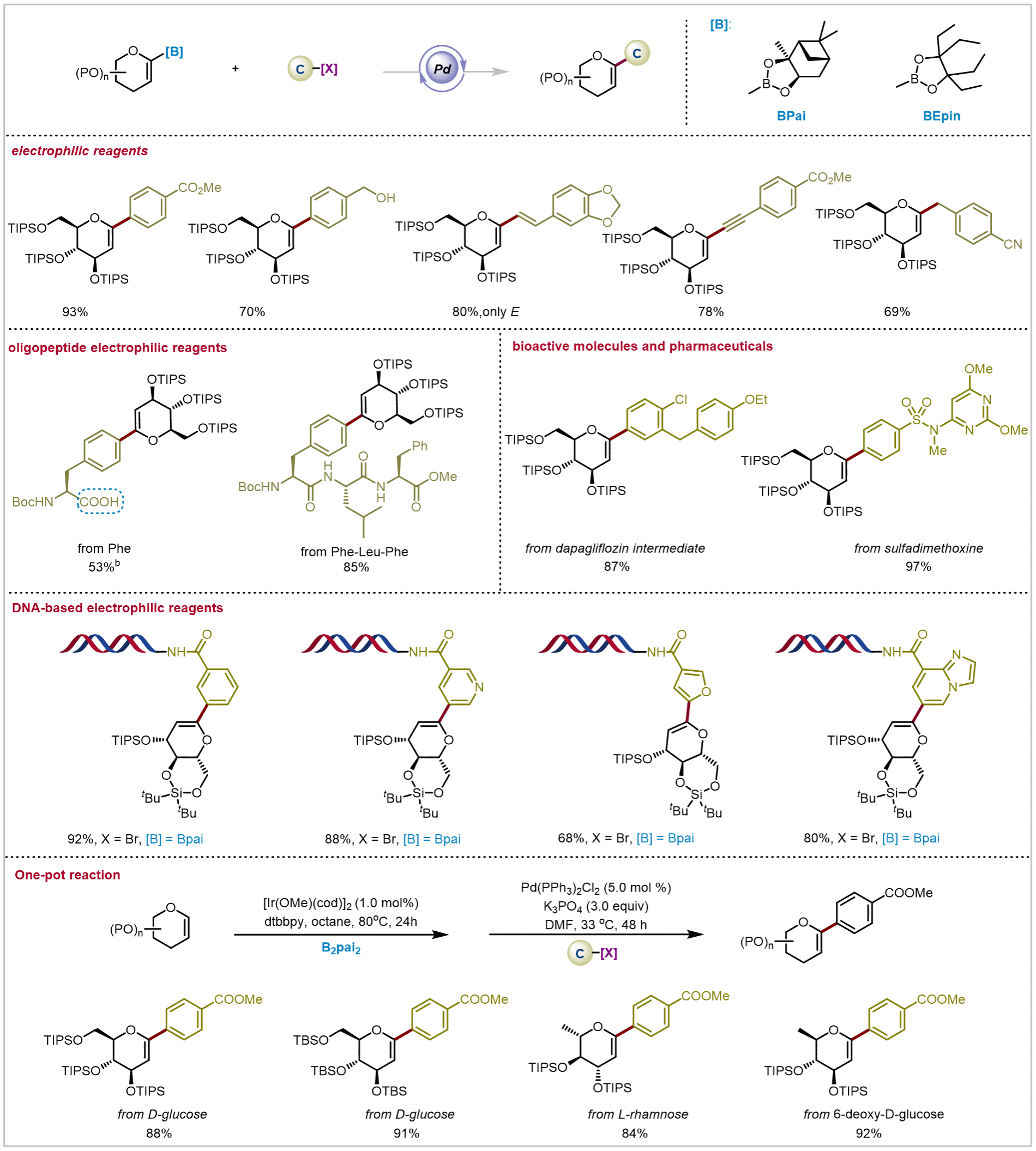
Figure 3: Reaction Substrate Expansion and One-Pot Synthesis
Finally, to explore the synthetic utility of this strategy, the authors conducted post-synthesis diversification experiments on the products (Figure 4). First, the synthesis of the antidiabetic drug molecules dapagliflozin and tolvogliflozin was achieved. Simultaneously, double bond transformations were performed on the synthesized drug-oligosaccharide products, yielding novel C-glycoside molecules with potential drug activity. Subsequently, through multi-step modifications of the reaction products, tolvogliflozin and its isomers were successfully synthesized, further demonstrating the practicality of the current approach.
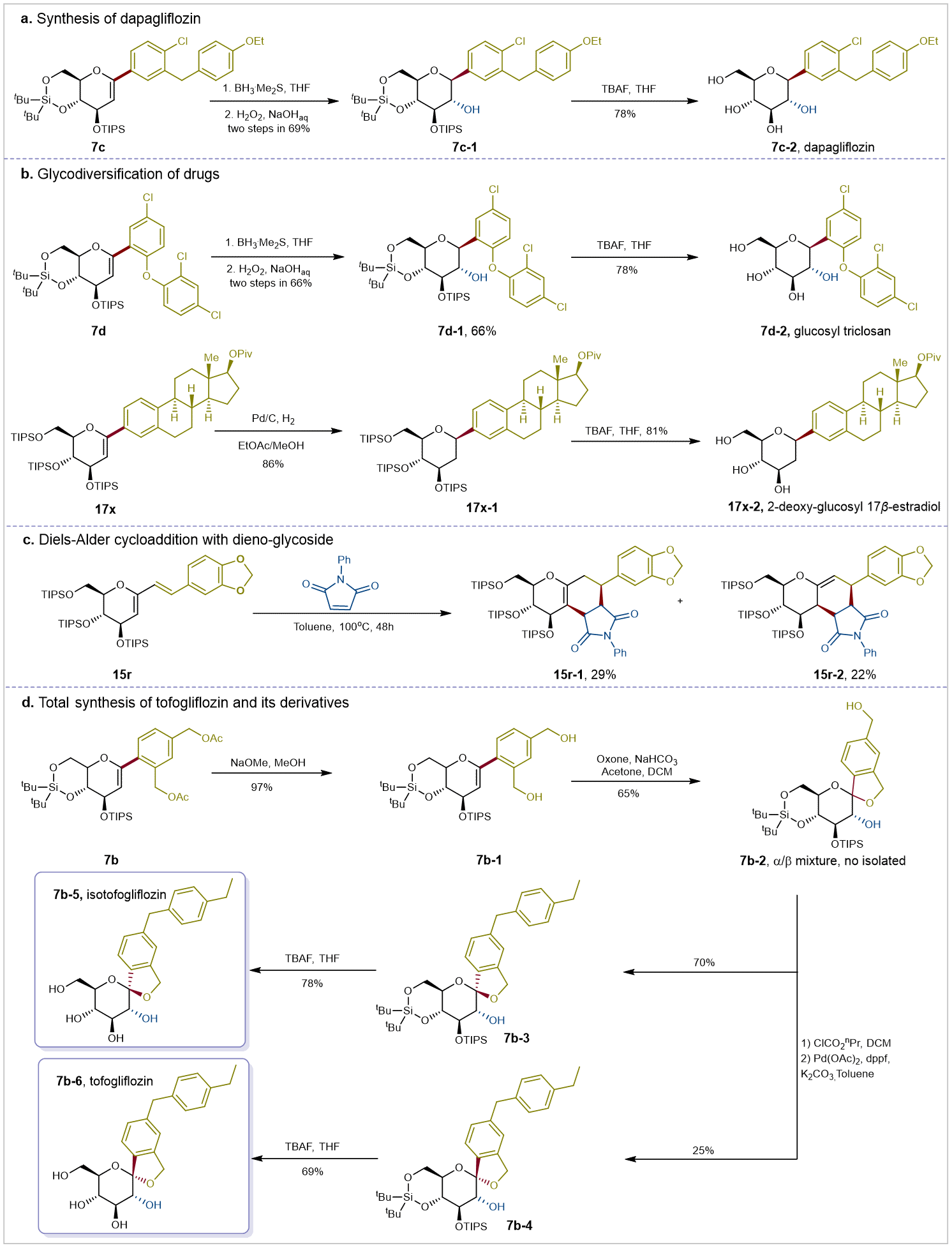
Figure 4: Late-stage Derivatization and Synthesis
In this work, the Zhu Feng group achieved a significant breakthrough in developing novel stable glycal boronate metal reagents, effectively addressing the reagent stability challenges in glycal-type Suzuki-Miyaura coupling reactions. This method exhibits outstanding reactivity, stability, versatility, and ease of operation, demonstrating broad potential for application in the preparation of C-glycoside drug molecules and natural products.
The research findings were recently published in Nature Communications under the title “Palladium-catalyzed Suzuki-Miyaura cross-couplings of stable glycal boronates for robust synthesis of C-1 glycals.” Chen Anrong, a doctoral student at the Center for Frontier Science in Transformative Molecules, served as the first author. Associate Professor Feng Zhu and Associate Researcher Lijuan Zhu are the co-corresponding authors. This research was supported by the Youth Scientist Program of the Key R&D Program of the Ministry of Science and Technology, the National Natural Science Foundation of China, the Shanghai Municipal Science and Technology Commission, and the Zhangjiang Advanced Research Institute of Shanghai Jiao Tong University.
Original link: https://doi.org/10.1038/s41467-024-49547-9
Author:
Zhu Feng Research Group






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn