搜索


Alpha-synuclein (α-Syn) is an intrinsically disordered protein highly expressed in presynaptic terminals. Its pathological aggregates serve as a key biomarker for Parkinson's disease and are closely associated with disease progression. Under physiological conditions, α-Syn promotes synaptic vesicle (SV) clustering via synaptotagmin (VAMP2) and regulates the assembly of the soluble N-ethylmaleimide-sensitive receptor complex (SNARE complex)¹. However, its physiological conformation and the molecular mechanisms governing its interactions with SVs and other associated proteins remain unclear. Additionally, whether and how α-Syn's physiological functions influence its pathological aggregation remains unknown.
On July 1, 2024, the research group led by Dan Li at the Center for Ultrafast Science, Zhangjiang Advanced Research Institute, Shanghai Jiao Tong University, in collaboration with the group led by Jiajie Diao at the University of Cincinnati College of Medicine, published their findings titled “VAMP2 chaperones α-synuclein in synaptic vesicle co-condensates” in Nature Cell Biology. The researchers first reported the phenomenon of co-phase separation of α-syn, VAMP2, and SV in vitro and in neurons. Subsequently, they discovered that the membrane-proximal region of VAMP2 binds to the carboxy-terminal region of α-syn through electrostatic interactions, mediating the dynamic assembly of multicomponent condensates that protect α-syn from forming pathogenic amyloid fibrils. This study not only elucidates the molecular mechanism by which α-synuclein performs its physiological functions through phase separation but also reveals a novel function of VAMP2: maintaining normal α-synuclein function and preventing its pathological aggregation by forming multicomponent condensed phases within presynaptic vesicles. This finding suggests that the failure of this mechanism may be closely associated with the pathogenesis of Parkinson's disease.
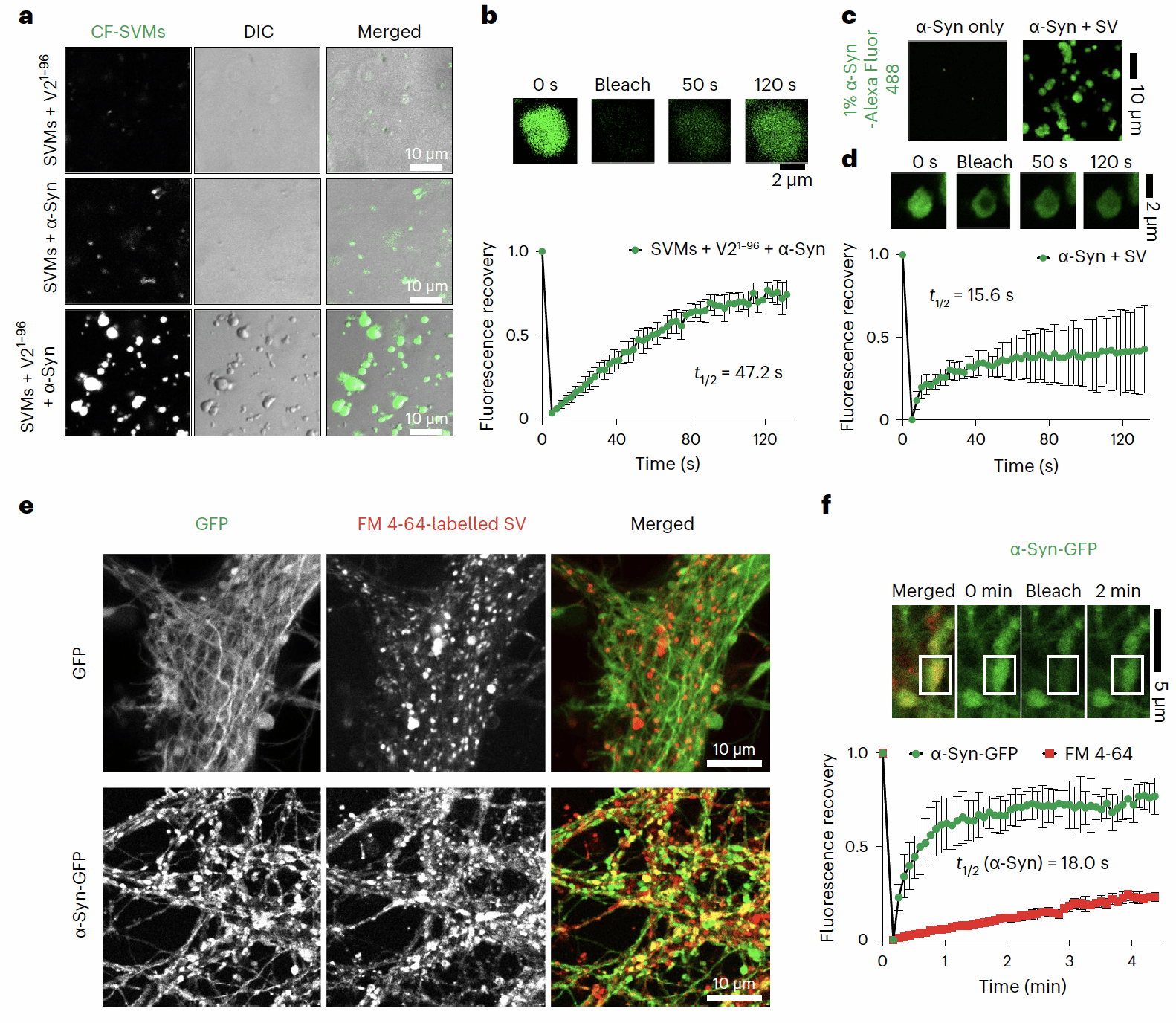
Figure 1. Multicomponent Liquid-Liquid Phase Separation of α-Syn, VAMP2, and SVs
The Li Dan research group has long focused on the dynamic regulatory mechanisms and chemical intervention studies of phase separation and aggregation of key pathological proteins in neurodegenerative diseases. Previous work systematically elucidated how diverse molecules (e.g., molecular chaperones, chemical small molecules, glycans, viruses) recognize and regulate the phase separation and aggregation of pathological proteins (e.g., α-syn in Parkinson's disease, FUS and TDP-43 in ALS)²,³,⁴. The group also developed small-molecule probes for specific recognition of α-syn aggregates for clinical disease detection⁵. The Li Dan research group initiated this project with collaborators as early as 2017. Starting from the liquid-liquid co-phase separation phenomenon involving multiple components (α-syn, VAMP2, SV), they progressively elucidated how VAMP2 and SV promote physiological functions by regulating the dynamic phase separation of α-syn. During this research, multiple studies reported that α-syn promotes localized nucleation and aggregation through liquid-liquid phase separation⁶, leading to the current understanding that α-syn phase separation is closely linked to its pathological toxicity. Additionally, SVs near the presynaptic membrane were found to form aggregates with synaptophysin⁷,⁸, creating clusters of presynaptic SVs that serve as a reserve pool for rapid neurotransmitter release. However, whether α-syn phase separation plays a role in its normal physiological functions and what role SVs play in this process remain key unresolved scientific questions in the field. In this work, researchers observed that α-syn not only undergoes phase separation on its own but also forms multicomponent co-phase separation with VAMP2 and SVs. Further, liquid-state NMR and cross-linking mass spectrometry characterized the structural basis of α-syn-VAMP2 interactions. Through designed mutants, the study validated that the negatively charged carboxy-terminal region of α-syn and the positively charged membrane-proximal region of VAMP2 constitute the critical interaction domains for multicomponent co-phase-separated aggregate formation. Subsequently, disruption of the α-syn-VAMP2 co-aggregating interaction was found to significantly reduce SV clustering, impair SNARE complex assembly in neurons, and decrease neurotransmitter transport efficiency. Finally, in vitro thioflavin T kinetics and cytotoxicity assays demonstrated that the VAMP2-α-syn interaction critically regulates suppression of α-syn amyloid pathology aggregation and its pathological toxicity.
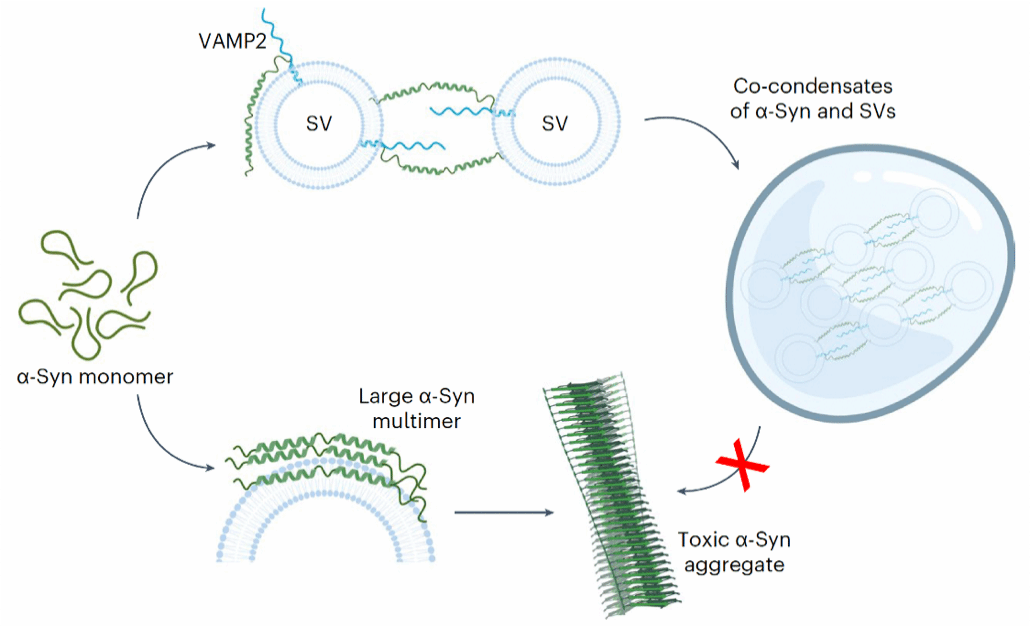
Figure 2. Schematic illustration of the molecular mechanism by which VAMP2 regulates α-syn phase separation and function
It is noteworthy that the research group led by Janin Lautenschläger at the University of Cambridge, UK, independently discovered a similar phenomenon of VAMP2 regulating α-syn phase separation at the same time⁹. These two independent studies mutually validated each other and were published back-to-back on the same day in Nature Cell Biology (Figure 3).
Figure 3. Another similar work published back-to-back
Dr. Wang Chuchu is the first author of this work. Professor Li Dan from the Center for Ultrafast Science at the Zhangjiang Advanced Research Institute of Shanghai Jiao Tong University and Professor Diao Jiajie from the University of Cincinnati College of Medicine are the co-corresponding authors of this paper.
Original link: https://doi.org/10.1038/s41556-024-01456-1
References:
1. Burré, J. et al. α-Synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329, 1663–1667 (2010).
2. Li D, Liu C. Conformational strains of pathogenic amyloid proteins in neurodegenerative diseases. Nat Rev Neurosci. (9):523-534 (2022).
3. Tao Y, et al. Heparin induces α-synuclein to form new fibril polymorphs with attenuated neuropathology. Nat Commun. 13(1):4226 (2022).
4. Tao Y, et al. Structural mechanism for specific binding of chemical compounds to amyloid fibrils. Nat Chem Biol. (10):1235-1245 (2023).
5. Xiang J, et al. Development of an α-synuclein positron emission tomography tracer for imaging synucleinopathies. Cell 186(16):3350-3367 (2023).
6. Ray, S. et al. α-Synuclein aggregation nucleates through liquid–liquid phase separation. Nat. Chem. 12, 705–716 (2020).
7. Chen, X. et al. Phase separation at the synapse. Nat. Neurosci. 23, 301–310 (2020).
8. Qiu, H. et al. Short-distance vesicle transport via phase separation. Cell 187, 2175–2193.e21 (2024).
9. Agarwal, A. et al. VAMP2 regulates phase separation of alpha-synuclein. Nat. Cell Biol. https://doi-org.stanford.idm.oclc.org/10.1038/s41556-024-01451-6 (2024).
Author:
Li Dan Research Group
Contributing Unit:
Center for Ultrafast Science






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn