搜索


Recently, a team led by Professor Qiu Huibin (School of Chemistry and Chemical Engineering) from the Center for Innovation in Synthetic Science at Shanghai Jiao Tong University's Zhangjiang Advanced Research Institute, in collaboration with the team of Distinguished Associate Professor Shen Chengshuo from the School of Chemistry and Chemical Engineering at Zhejiang Sci-Tech University, reported a non-covalent porous crystal constructed based on low-symmetry spiroene. This crystal ingeniously utilizes the self-recognition properties between spiroene molecules, achieving multi-level assembly to ultimately form a stable non-covalent porous framework. Notably, this framework material is entirely held together by π interactions, exhibiting outstanding thermal stability, ease of preparation, and recyclability. Its unique pore environment also enables exceptional selective adsorption of organic small molecules.
This work, titled “Facile Fabrication of Recyclable Robust Noncovalent Porous Crystals from Low-Symmetry Helicene Derivative,” was published in Nature Communications.
Porous materials, characterized by low density and high specific surface area, find extensive applications in adsorption, catalysis, energy storage, and sensing, making them a current research hotspot. Among these, porous framework materials constructed from stable chemical bonds such as covalent and coordinate bonds—including covalent organic frameworks (COFs) and metal-organic frameworks (MOFs)—possess stable and fixed porous structures. However, such materials typically exhibit poor solubility, limiting their processing and recyclability. In recent years, non-covalent porous materials held together by intermolecular interactions such as hydrogen bonds, π interactions, and polar interactions have garnered increasing attention due to their superior solubility and processability. Despite progress in this field, structural design remains unsystematic, and the weak interactions sustaining these porous structures are inherently less robust than chemical bonds, significantly compromising material stability. Therefore, designing easily preparable and structurally stable noncovalent porous crystals represents a major challenge in this field.
Researchers discovered that using low-symmetry monosubstituted [6]spiroene derivatives (D6H) as fundamental building blocks, combined with a modular self-assembly approach, enables the successful construction of noncovalent porous frameworks with one-dimensional through-hole structures. As shown in Figure 1a, racemic D6H molecules undergo primary assembly via chiral self-recognition, forming secondary structures. During this process, P and M enantiomers selectively bind to molecules of the same chirality, assembling into a helical pillar secondary structure with a three-molecule periodicity. The helical chirality of the secondary structure aligns with that of the spiroene building blocks. Subsequently, these helix columns from primary assembly selectively assemble with enantiomeric helix columns. Specifically, three P-chiral helix columns interlock with three M-chiral helix columns, mutually stacking to form a one-dimensional pore structure, ultimately constituting an ordered noncovalent porous framework.
Leveraging abundant intermolecular π interactions, this noncovalent porous crystal exhibits exceptional stability. As shown in Figure 1c, its X-ray diffraction signal remains virtually unchanged during continuous heating from room temperature to 275 °C. This indicates that even under high-temperature conditions, solvent molecule desorption does not cause pore collapse, maintaining the stability of its porous structure. Furthermore, this noncovalent porous framework is easily prepared and recyclable. Porous materials can be synthesized simply, rapidly, and in large quantities via rotary evaporation. As shown in Figure 1d, the porous powder obtained by rotary evaporation of dichloromethane solution exhibits a microcrystalline structure under scanning electron microscopy despite its fine particle size. It shares the same hexagonal prismatic structure as the single crystals obtained through slow evaporation, demonstrating identical porous properties.
The porous powder obtained via rotary evaporation exhibits adsorption properties after activation to remove residual solvents from the pores. Furthermore, the relatively mild π-electron environment formed by the π-conjugated structure of the one-dimensional pore distribution enables screening of small-molecule polarity, selectively adsorbing a series of small molecules with matching polarity. As shown in Figure 1e, thermogravimetric analysis of porous powders exposed to different solvent vapors indicates that activated porous powders exhibit superior adsorption capacity for ethers and chlorinated hydrocarbons. Conversely, they demonstrate reduced adsorption for less polar cyclohexane and more polar methanol, revealing distinct adsorption preferences toward solvents of varying polarity. Notably, the activated porous powder maintains stable porosity throughout adsorption. Repeated adsorption-desorption cycles preserve both its adsorption capacity for tetrahydrofuran and its original microcrystalline morphology (Figure 1f).
Leveraging this adsorption selectivity, researchers subsequently exposed the porous material to dioxane and cyclohexane vapors. NMR hydrogen spectroscopy confirmed distinct adsorption behaviors between the two solvents (Figure 1g). The porous powder exhibited notable adsorption capacity for dioxane, which matches its polarity, while showing negligible adsorption for the low-polarity cyclohexane. Furthermore, when the activated porous powder was exposed to a mixed vapor generated from an equimolar mixture of DIOX and cyclohexane, the adsorption capacity for DIOX significantly exceeded that for cyclohexane. This result further demonstrated the porous powder's ability to selectively adsorb DIOX.
In summary, this study introduces a novel design strategy for constructing noncovalent porous frameworks. Within this approach, low-symmetry building units undergo multilevel assembly through abundant noncovalent π interactions to form structurally ordered porous frameworks. The aforementioned noncovalent porous materials demonstrate broad application prospects across multiple fields, holding promise for increasingly diverse practical applications in the future.
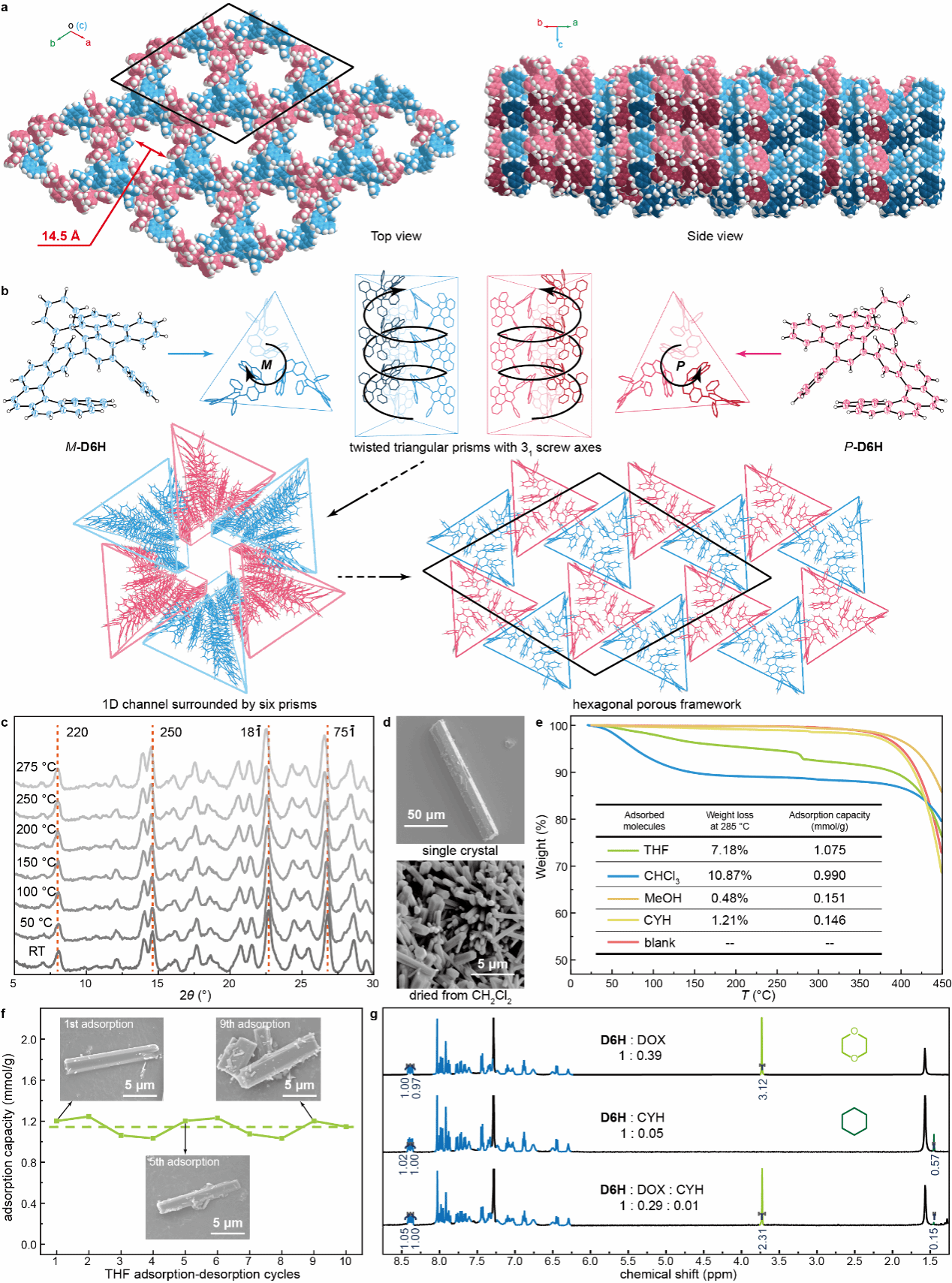
Figure 1 a) Space-filling model of the crystal and its one-dimensional pore structure; b) Schematic illustration of the porous framework formed through multi-level self-assembly based on chiral self-recognition; c) Variable-temperature X-ray diffraction patterns of the porous crystal from room temperature to 275 °C; d) scanning electron micrographs of the single crystal and powder obtained by rotary evaporation from a dichloromethane solution; e) thermogravimetric analysis curves and corresponding calculated adsorption capacities of the activated porous powder material after 48 h adsorption in different solvent atmospheres; f) adsorption capacity curve from adsorption-desorption cycling experiments with tetrahydrofuran, along with scanning electron micrographs of samples taken during the cycling process; g) NMR hydrogen spectra of the activated porous powder material after 48 h adsorption in dioxane, cyclohexane, and mixed vapor of both solvents.
Zhang Guoli, a doctoral candidate at the School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, is the first author of this paper. Professor Qiu Huibin (School of Chemistry and Chemical Engineering) from the Center for Innovation in Synthetic Science at the Zhangjiang Advanced Research Institute, Shanghai Jiao Tong University, and Shen Chengshuo, Distinguished Associate Professor at the School of Chemistry and Chemical Engineering, Zhejiang University of Science and Technology, are the corresponding authors. This work was supported by the National Natural Science Foundation of China, the Ministry of Science and Technology, the Shanghai Municipal Science and Technology Commission, the Shanghai Municipal Education Commission, Shanghai Jiao Tong University, the Zhangjiang Institute for Advanced Studies at Shanghai Jiao Tong University, the Zhejiang Natural Science Foundation, and Zhejiang University of Science and Technology.
Paper link: https://doi.org/10.1038/s41467-024-49865-y
Author:
Zhang Guoli
Contributing Unit:
Center for Innovation in Synthetic Science (Synthetic Chemistry Platform)






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn