搜索


Research Background:
The rapidly growing energy demands of modern society urgently require the development of high-performance rechargeable batteries. Anode-less lithium metal batteries, which eliminate the need for any lithium metal during production, have garnered significant attention in recent years due to their high energy density, manufacturing convenience, and low cost. Despite numerous efforts to further enhance energy density, a long-overlooked issue lies in the “inactive component”—the copper foil current collector (Cu), which accounts for 25% of the anode-less battery's total mass without contributing any capacity. Therefore, replacing traditional copper foil with lightweight components holds promise for significantly boosting the energy density of anode-less batteries. Sputtering an ultra-thin copper layer onto a polymer substrate as a composite current collector can effectively reduce weight and enhance the energy density of anode-less batteries. However, existing composite current collectors suffer from poor interface stability, where the copper layer tends to separate from the polymer substrate, limiting the electrochemical performance and safety of anode-less batteries. The core challenge lies in the typically weak interaction between conventional polymer substrates and copper layers, resulting in high sheet resistance and poor structural stability for composite current collectors. This limits the reversibility and kinetics of lithium metal deposition-stripping processes, severely hindering performance improvements and rapid iteration of anode-less batteries.
In response, a team led by Sun Hao—Associate Professor at the Center for Frontier Science in Transformative Molecules, Shanghai Jiao Tong University, and a resident scientist at the Zhangjiang Advanced Research Institute—developed an ultralight composite current collector. With a thickness of just 5.2 μm and a surface density as low as 0.78 mg cm⁻², it achieves a weight reduction of 49–91% compared to copper-based current collectors in high-energy-density lithium batteries (Figure 1), while boosting energy density by 36–61%. Simultaneously, the nanostructured copper layer on the composite current collector surface reduces the nucleation overpotential of the battery, significantly improving the adsorption, nucleation, and deposition processes of lithium ions at the “composite current collector-electrolyte” interface. It can still stably cycle for 50 cycles under a current density of 10 mA cm⁻², enhancing the reversibility and kinetics of the lithium metal deposition-stripping process. By modulating the molecular structure of the polymer substrate polyamide carbamide (PASC) to establish coordination interactions with the Cu deposition layer, the battery achieves outstanding interfacial stability, flexibility, and safety without a negative electrode.
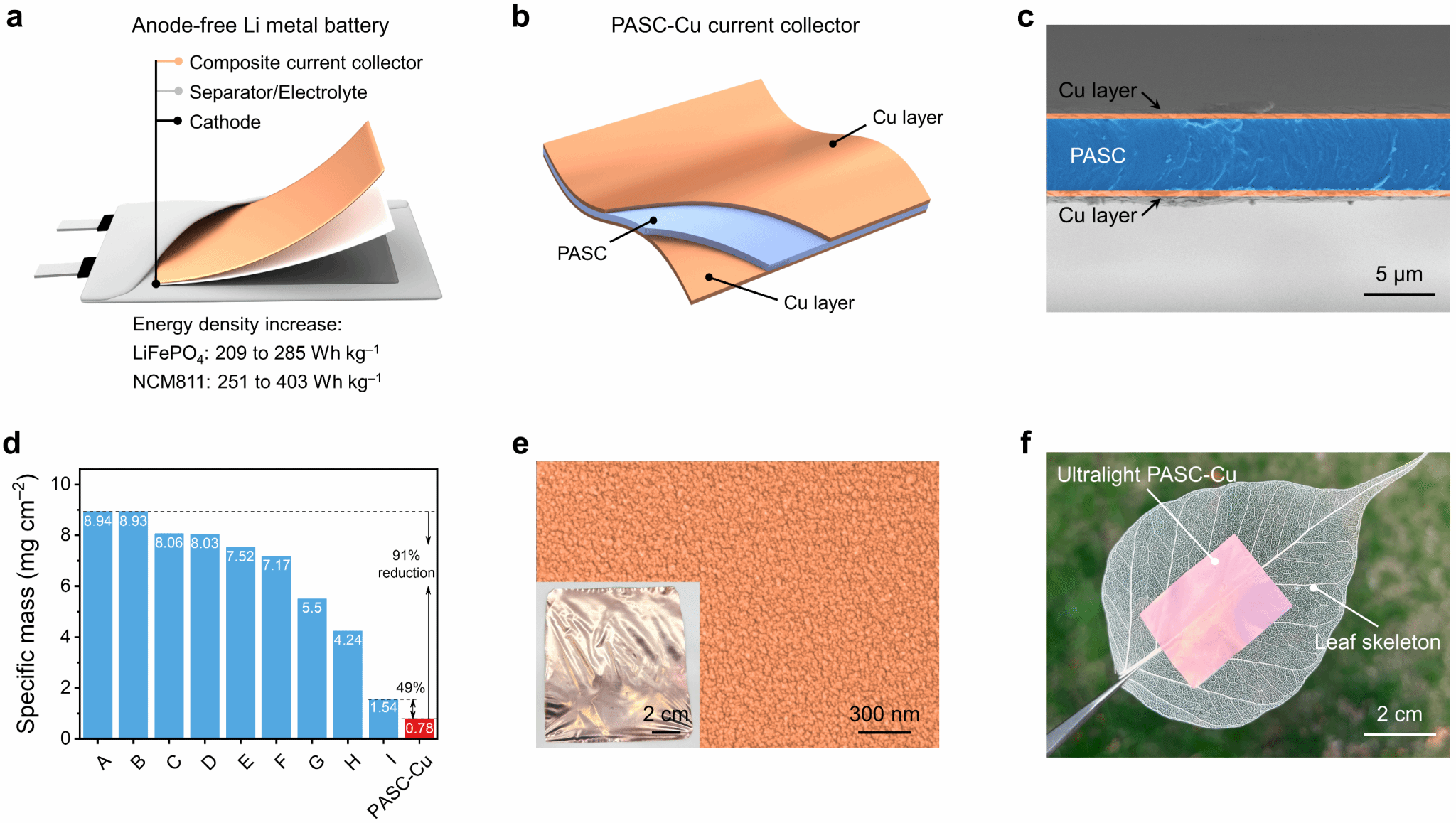
Figure 1. An ultralight composite current collector enabling high-energy-density anode-free lithium metal batteries
The research findings were published in Advanced Materials under the title “An Ultralight Composite Current Collector Enabling High-Energy-Density and High-Rate Anode-Free Lithium Metal Battery.” The first authors are doctoral candidates Ouyang Zhaofeng and Wang Shuo, along with postdoctoral researcher Wang Yan. Associate Professor Sun Hao served as the corresponding author. This work received substantial support from the National Natural Science Foundation of China, the Special Fund for Basic Research of Central Universities, the Center for Transformative Molecular Frontier Science at Shanghai Jiao Tong University, and the Zhangjiang Institute for Advanced Studies.
Research Highlights:
The team first characterized the crystal structure of the surface Cu layer via X-ray diffraction (XRD), confirming successful PASC-Cu synthesis (Figure 2a). Electrical testing revealed that as the surface Cu layer thickness decreased, PASC-Cu exhibited increased resistivity and decreased conductivity. To maximize energy density in anode-free batteries, subsequent testing was conducted using a 100 nm Cu layer (Figure 2b). Additionally, PASC-Cu exhibited a bond strength of 3.9 MPa, significantly surpassing polyurethane (PU, 2.1 MPa) and polyethylene terephthalate (PET, 1.5 MPa) (Figure 2c). Subsequent detailed investigation of the interaction between the PASC substrate and the Cu layer involved uniformly mixing PASC with Cu, followed by X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FTIR), and Raman spectroscopy (Raman) analyses (Fig. 2d-f). Results revealed strong coordination interactions between N–H and C=O groups on PASC and the Cu layer, conferring excellent interfacial stability to the composite current collector. After 1400 bending cycles, its resistance remained stable, and the surface Cu layer retained its dense and uniform structure (Fig. 2g, i), laying the foundation for flexible wearable applications of flexible anode-free batteries.
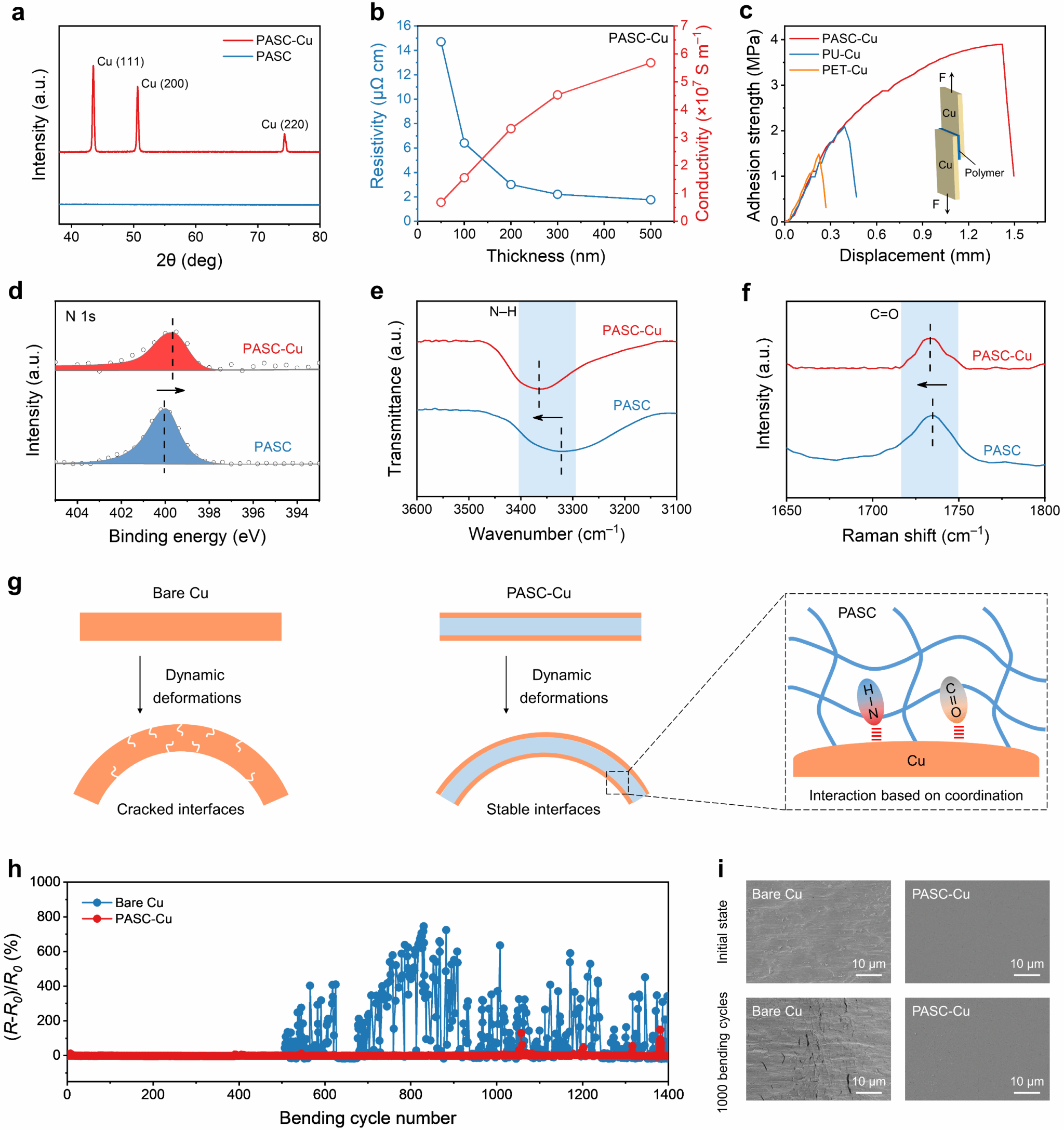
Figure 2. Coordination Analysis of PASC with Cu
By assembling a Li/PASC-Cu half-cell, it was found that the nanostructured surface of the composite current collector effectively reduced the nucleation overpotential (47.8 mV), significantly improving the adsorption and nucleation processes of lithium ions at the “composite current collector-electrolyte” interface. This enabled stable and reversible deposition-stripping behavior (Figure 3a, b). Under conditions of 1 mA cm⁻² and 1 mAh cm⁻², the average coulombic efficiency after 200 cycles was approximately 98.5%. Even under extreme conditions of 10 mA cm⁻² and 2 mAh cm⁻², reversible deposition/stripping behavior was achieved for 50 cycles (Fig. 3c, d). Scanning electron microscopy (SEM) and laser confocal microscopy revealed uniform, dense lithium metal deposition layers on the PASC-Cu composite current collector across varying current densities and areal capacities (Fig. 3e–g). First, the nanostructured Cu layer of the PASC-Cu composite current collector provides abundant nucleation sites, reducing overpotential and local current density during lithium deposition, thereby enabling uniform lithium nucleation and dense lithium plating. Second, compared to pure Cu foil, PASC-Cu exhibits superior electrolyte wettability, facilitating lithium ion transport and distribution, with an exchange current density of 0.201 mA cm−2 (Figure 3h). Furthermore, the high toughness of PASC enables effective stress dissipation to accommodate volumetric changes during lithium deposition, ensuring dense and uniform lithium plating even under high-rate conditions. Electrochemical impedance spectroscopy (EIS) results reveal no significant increase in interfacial impedance after 50 cycles for the half-cell based on the composite current collector (Figure 3h, i).
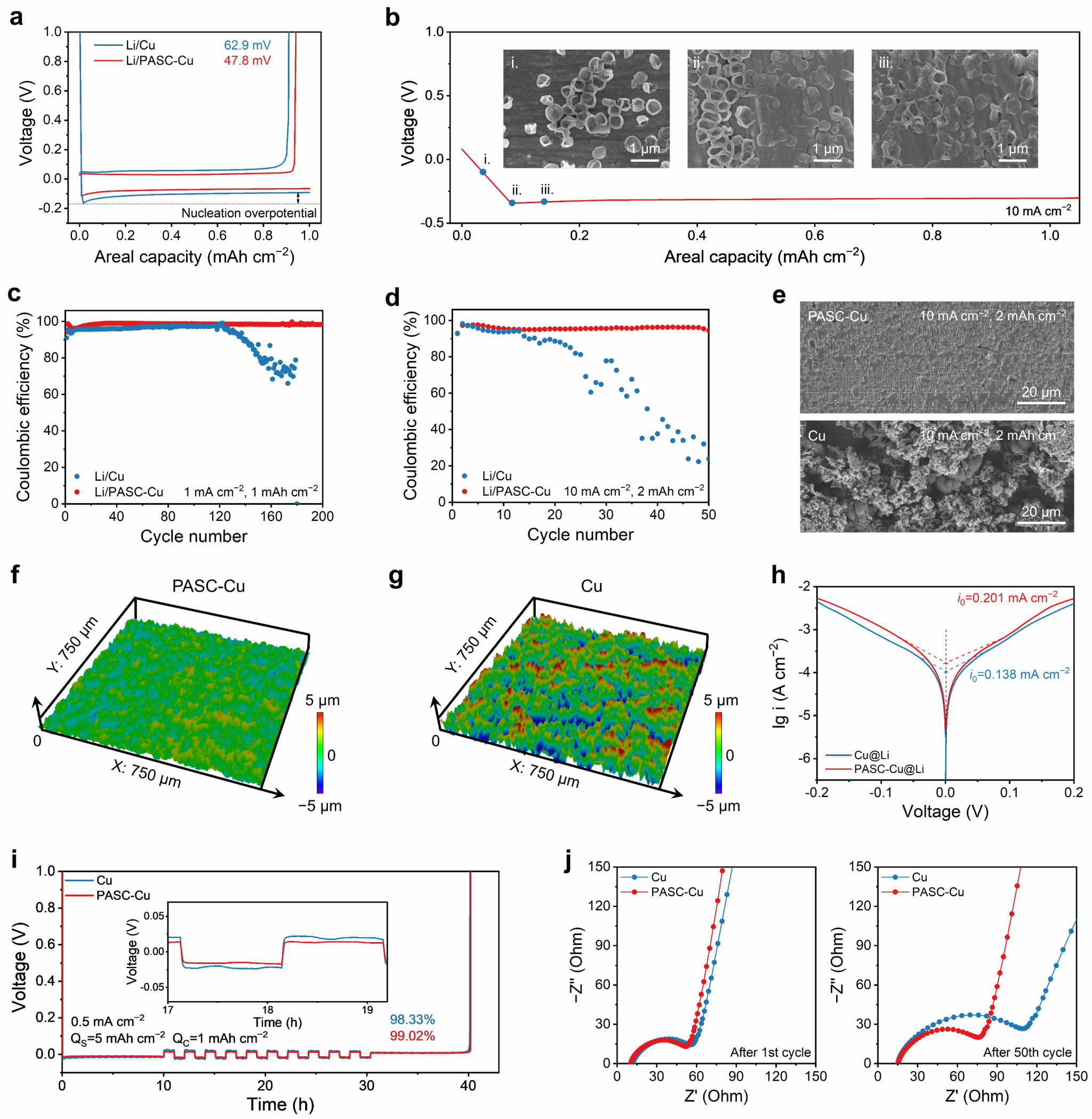
Figure 3. Electrochemical performance and lithium metal deposition morphology of Li/PASC-Cu half-cell
An anode-free battery (surface capacity 2.8 mAh cm−2) based on a PASC-Cu composite current collector and a lithium iron phosphate cathode exhibits superior cycling and rate performance. The battery maintains an average coulombic efficiency of 99.1% after 200 stable cycles and a capacity retention of 65.1% at a 5C rate (Fig. 4a, b). Subsequently, under low-electrolyte conditions (2 g Ah^(−1)), the energy density and power density of the anode-free battery were calculated to be 285 Wh kg^(−1) and 1666 W kg^(−1), respectively, based on the mass of all components including cathodes, anodes, current collectors, separators, and electrolytes (Fig. 4c, d). Using high-voltage NiCoMn ternary cathode materials, the all-component energy density of the anode-free battery reached 403 Wh kg^(−1). If subsequent studies further increase cathode loading while reducing electrolyte volume, the energy density of a 10 Ah anode-less battery based on the PASC-Cu composite current collector could reach 661 Wh kg^(−1), potentially meeting the energy density requirements for aircraft electrification (Fig. 4e, f).
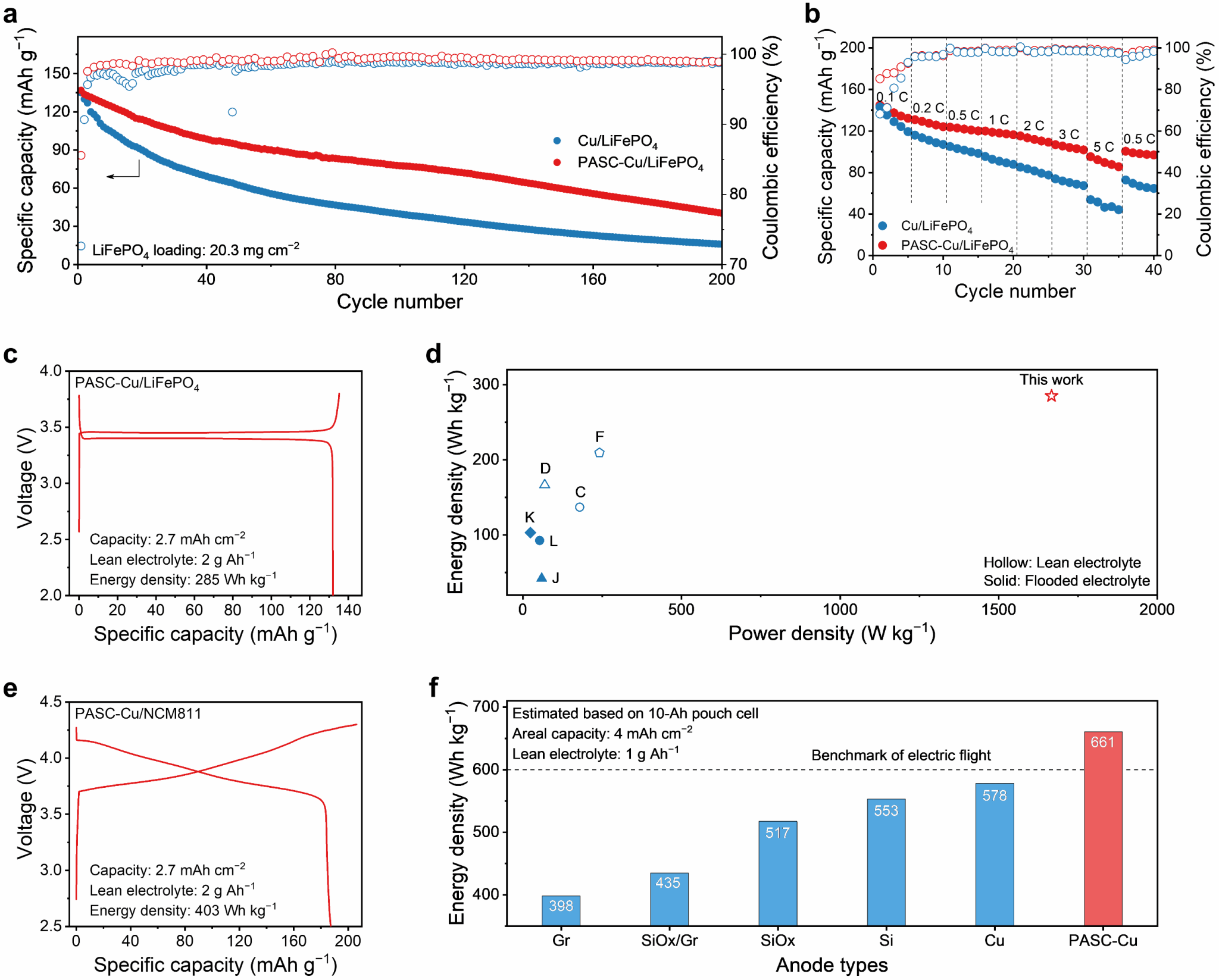
Figure 4. Electrochemical performance characterization of an anode-free battery based on the PASC-Cu composite current collector
Due to the excellent interfacial stability of the PASC-Cu composite current collector, the research team thoroughly investigated the flexibility of the anode-less pouch battery. Results show that even under dynamic deformations such as bending and folding, the PASC-Cu/LiFePO₄ pouch battery maintains a high capacity retention rate of 96.7%, whereas the Cu/LiFePO₄ battery achieves only 68.4% (Figure 5a). As demonstrated, the anode-free PASC-Cu/LiFePO₄ battery maintained a discharge voltage of 3.4 V under various deformations and continuously powered a light-emitting diode (Figure 5b). Additionally, the research team conducted a puncture test on anode-free pouch batteries using Cu foil and PASC-Cu composite current collectors. Results showed that the Cu/LiFePO₄ pouch battery short-circuited instantly upon puncture, with temperature immediately rising to 115°C (Figure 5c). In stark contrast, the PASC-Cu/LiFePO₄ pouch battery maintained stable voltage during puncture with no significant temperature increase (Figure 5d). This behavior stems from two key factors: First, the high toughness of PASC and its strong interfacial interaction with the Cu layer enable the Cu layer to tightly bond with the PASC substrate after penetration, preventing direct contact with the penetrating nail and thus avoiding short-circuiting. On the other hand, partial dissociation of the dynamic acylcyanuric acid (ASC) group at elevated temperatures transforms PASC into a flowable oligomer (Mn ≈ 8600 g mol–1). These oligomers infiltrate the interstices between Cu nanoparticles to block conductive pathways, thereby preventing localized thermal runaway and conferring exceptional safety to the anode-less battery.
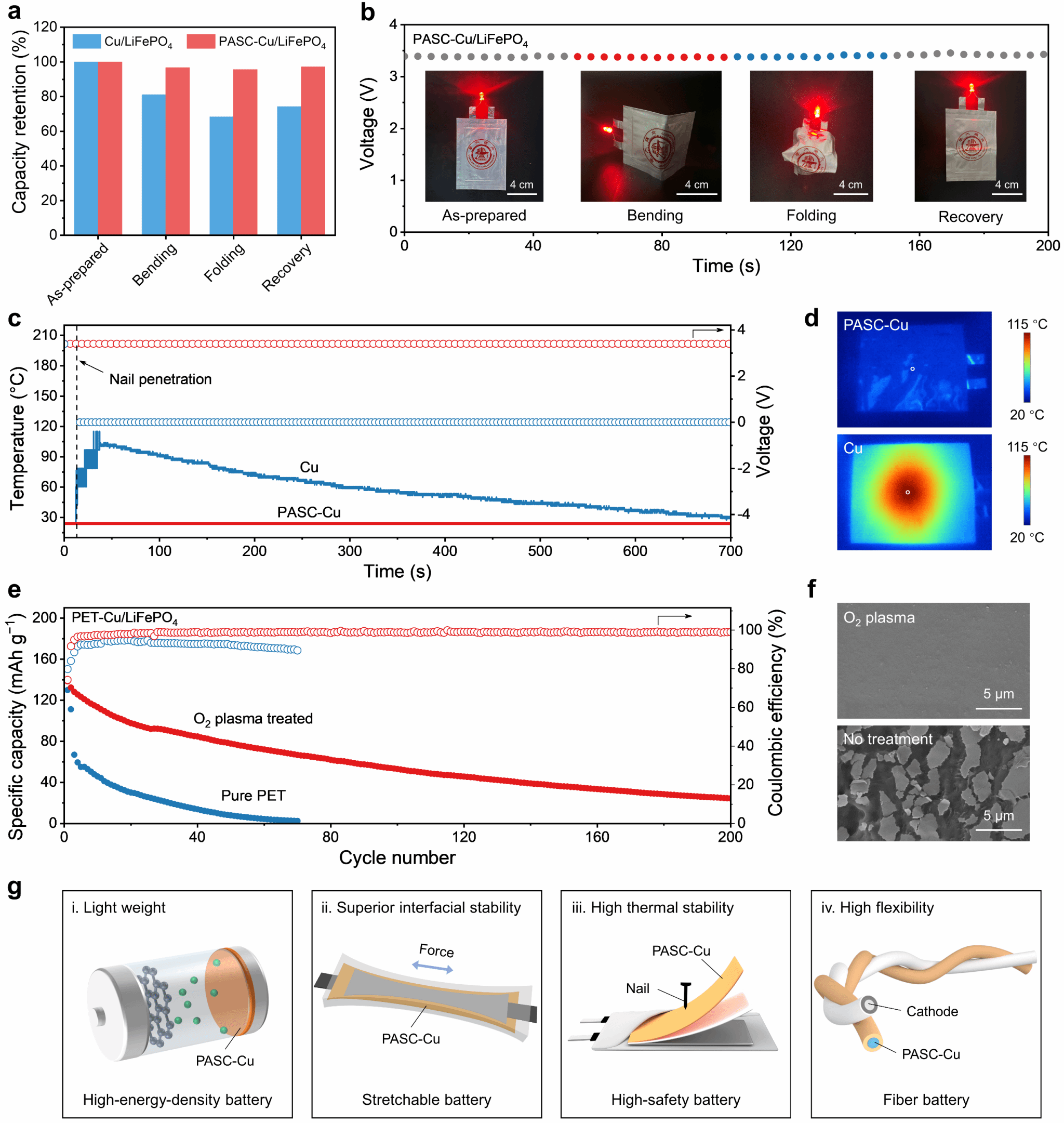
Figure 5. Demonstration of the Application and Universality of Anode-Free Batteries Based on PASC-Cu Composite Current Collectors
In summary, this work developed an ultralight composite current collector with an areal density of only 0.78 mg cm⁻². The assembled anode-free battery achieved energy and power densities of 403 Wh kg⁻¹ and 1666 W kg⁻¹, respectively, representing a 36–61% improvement over existing work. By modulating the molecular structure of polyamide-based urea, enhanced coordination with the copper deposition layer was achieved. The outstanding interfacial stability endows the anode-less battery with exceptional flexibility and safety, maintaining a capacity retention rate as high as 96.7% under dynamic deformations such as bending. Even after needle penetration testing, the battery exhibits low thermal runaway, effectively preventing thermal instability. Furthermore, the nanostructured surface of the composite current collector enables more uniform lithium-ion flow, achieving dense and uniform lithium metal deposition at 10 mA cm⁻². This significantly enhances the reversibility and kinetics of lithium metal deposition/stripping, synergistically improving the cycle stability and rate performance of the anode-free battery. This approach offers new insights for developing high-energy-density, high-rate, highly safe, and wearable electrochemical energy storage devices.
Original link: https://doi.org/10.1002/adma.202407648
Author:
Sun Hao's Team






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn