搜索


Recently, the internationally renowned materials journal Advanced Materials published online the latest research findings from Professor Xiaodong Zhuang of the Center for Innovation in Synthetic Science at the Zhangjiang Advanced Research Institute and Professor Xi Zhang of the School of Mechanical Engineering. Their study, titled “A Porous Li–Al Alloy Anode toward High-Performance Sulfide-Based All-Solid-State Lithium Batteries,” Through simple mechanical mixing and cold-pressing techniques, the study fabricated a porous and loose Li–Al alloy anode (LiAl-p) and applied it to sulfide all-solid-state batteries.
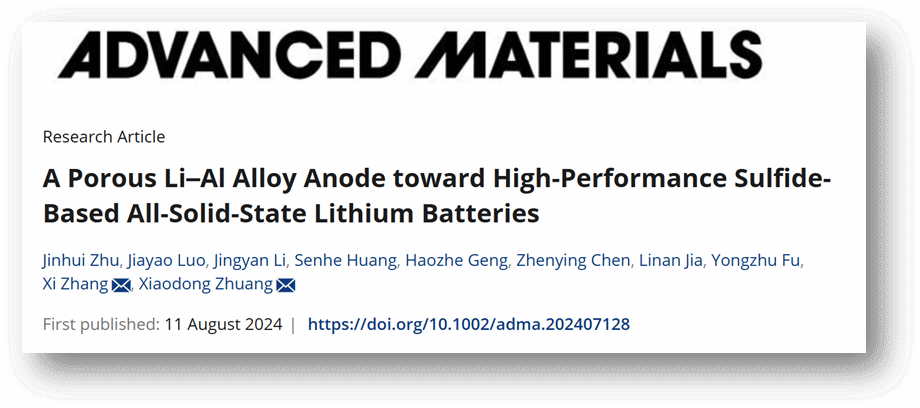
This porous lithium-aluminum alloy anode-based sulfide all-solid-state battery achieves a record-breaking critical current density at room temperature (50 mA/cm²), whereas conventional sulfide electrolyte lithium metal symmetric cells typically do not exceed 1 mA/cm², demonstrating significant application advantages. Furthermore, the all-solid-state battery with a porous lithium-aluminum alloy anode exhibits ultra-long-term stability (5000 h symmetric battery cycling) and areal capacity (NCM full cell: 11.9 mAh/cm²), whereas conventional lithium metal symmetric batteries typically demonstrate stability not exceeding 100 hours. Characterization via in situ Raman spectroscopy, theoretical calculations, TOF-SIMS, and FIB-SEM confirms that its porosity effectively mitigates volume expansion: 17% (porous Li–Al alloy in this paper) vs. 66% (dense Li–Al alloy) vs. 125% (aluminum anode), playing a crucial role in maintaining interface stability.
Compared to the dense Li–Al alloy anode (LiAl-d), LiAl-p exhibits smaller volume changes during lithium insertion/extraction (66% vs. 17%) (Figure 2). In situ Raman spectroscopy and molecular dynamics simulations reveal high chemical/electrochemical stability between the Li–Al alloy anode and SSE, while oxygen-containing groups remaining on the Li–Al alloy surface react with SSE to form a stable SEI (Figure 3). The symmetric cell based on LiAl-p achieves a record-breaking critical current density (6.0 mA/cm²) and lithium insertion/extraction stability (5000 h) (Figure 4). Post-cycle analysis of LiAl-p via surface composition (TOF SIMS) and internal structure (FIB-SEM, XCT) revealed a robust SEI rich in lithium-containing inorganic compounds on its surface. Its porous structure effectively mitigated volume effects and suppressed powdering (Figure 5). Finally, an all-solid-state battery based on LiAl-p and NCM811 was constructed, exhibiting an initial specific capacity of 200 mAh g^(−1) (0.1 C) and a capacity retention of 83% after 1800 cycles at 1 C. The maximum areal capacity of this full cell reached 11.9 mAh/cm², surpassing the highest reported areal capacity of sulfide all-solid-state batteries based on LMA and alloy anodes (Figure 6).
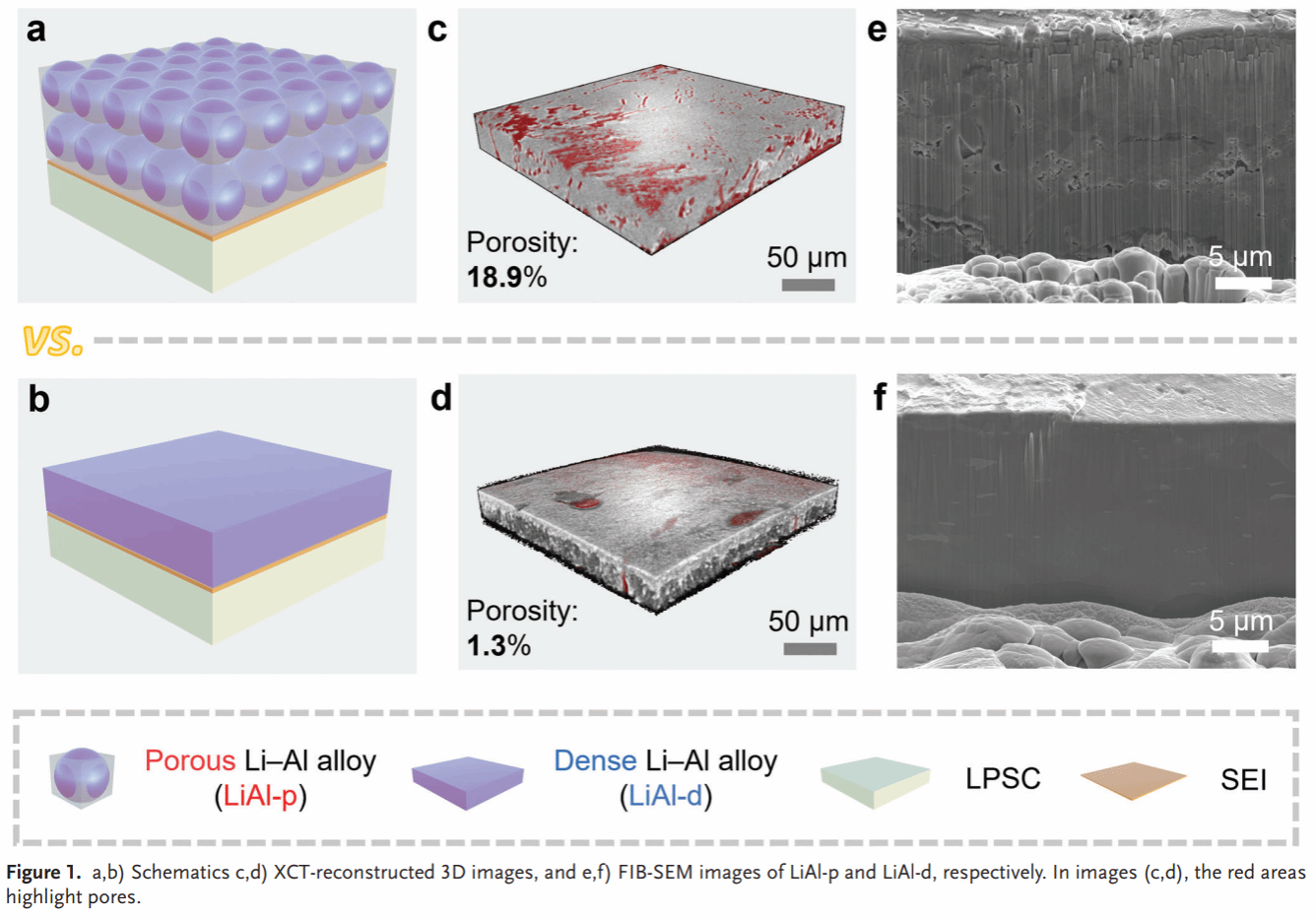
Figure 1. Schematic diagrams of LiAl-p and LiAl-d (a, b); XCT-reconstructed 3D images (c, d); and FIB-SEM images (e, f).
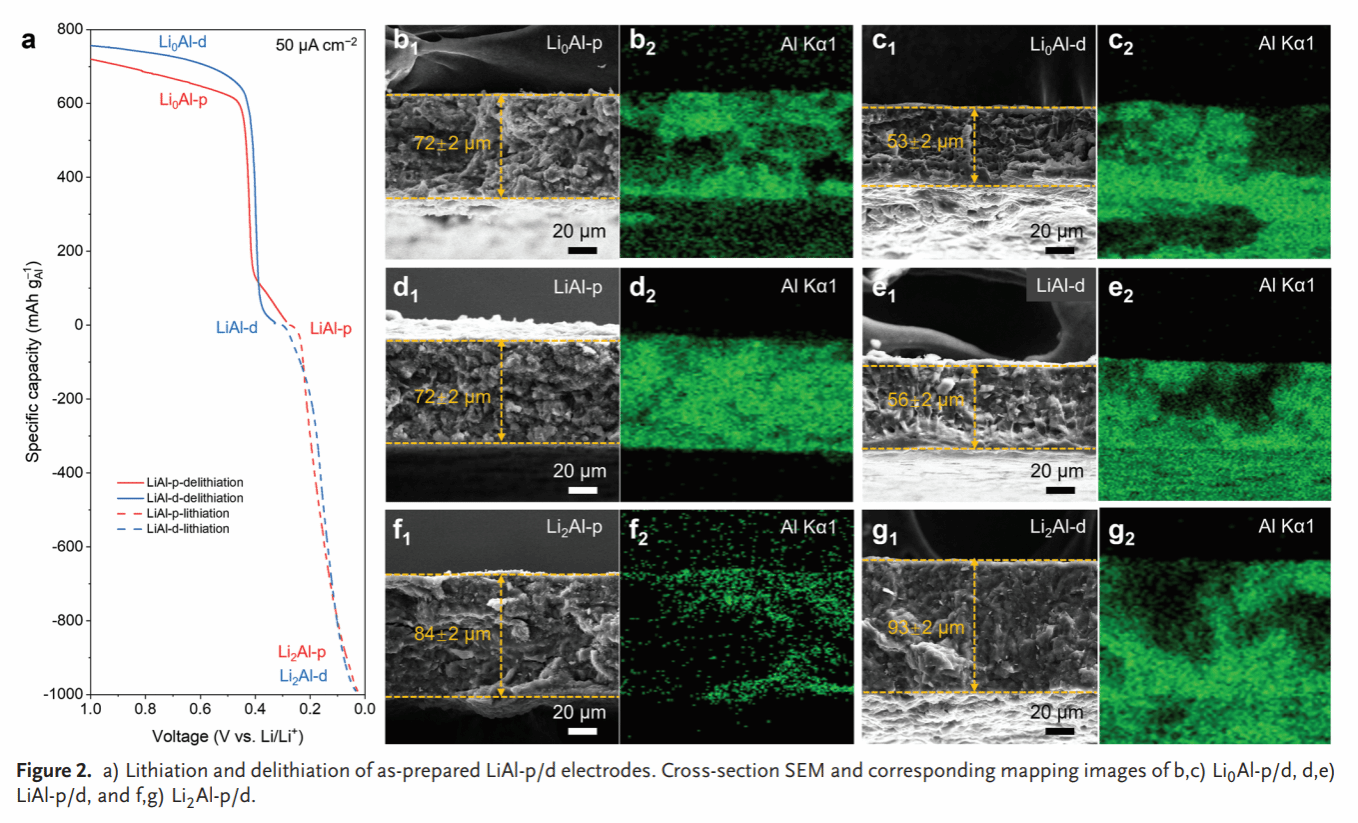
Figure 2. Lithium deintercalation curve of the LiAl-p/d electrode (a). Cross-sectional SEM images of the LiAl-p/d electrode in its pristine state, deintercalated state, and intercalated state (b–g).
Figure 3. In situ Raman spectrum at the LiAl-p/SSE interface (a). Theoretical molecular dynamics simulations at the LiAl-p/SSE interface (b–i).
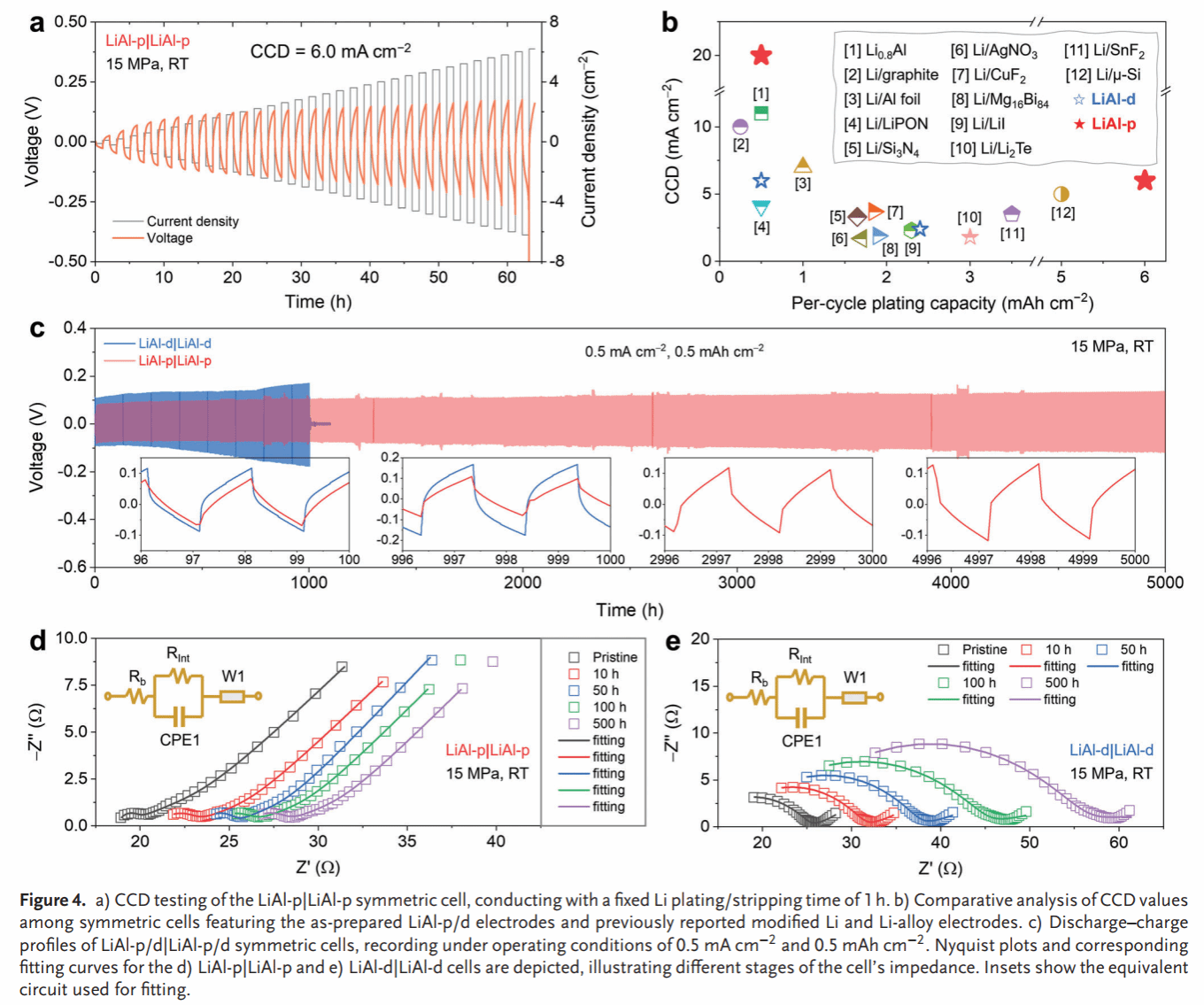
Figure 4. Critical current density of the LiAl-p symmetric cell (a) and comparison with literature-reported values (b). Long-term lithium insertion/extraction cycling of the LiAl-p/d symmetric cell (c), along with EIS spectra at different cycling stages (d, e).
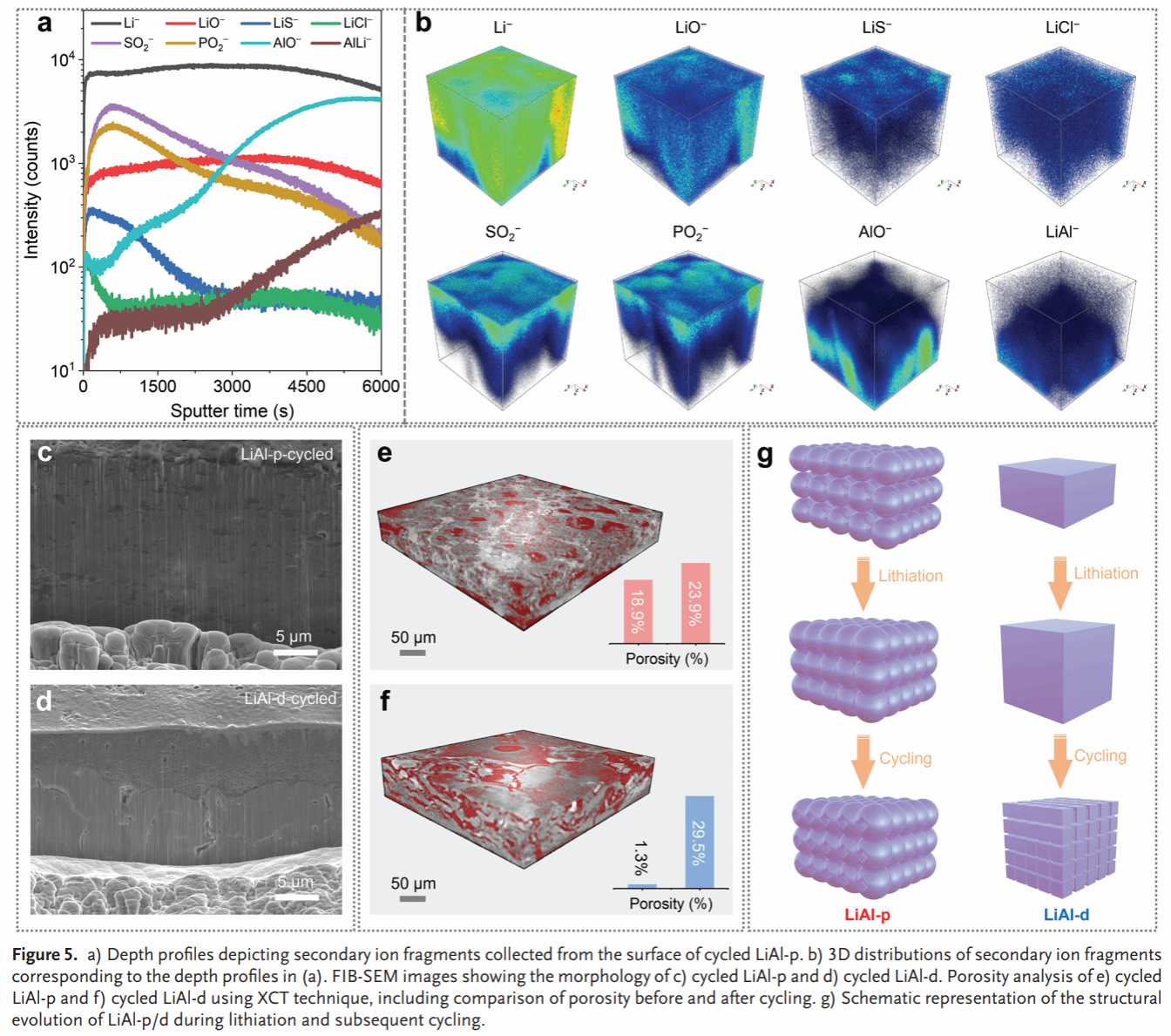
Figure 5. Characterization of LiAl-p after cycling: TOF SIMS spectrum (a) and 3D reconstruction (b). FIB-SEM images (c, d) and XCT images (e, f) of LiAl-p and LiAl-d after cycling. Schematic illustration of structural changes in LiAl-p/d during cycling (g).
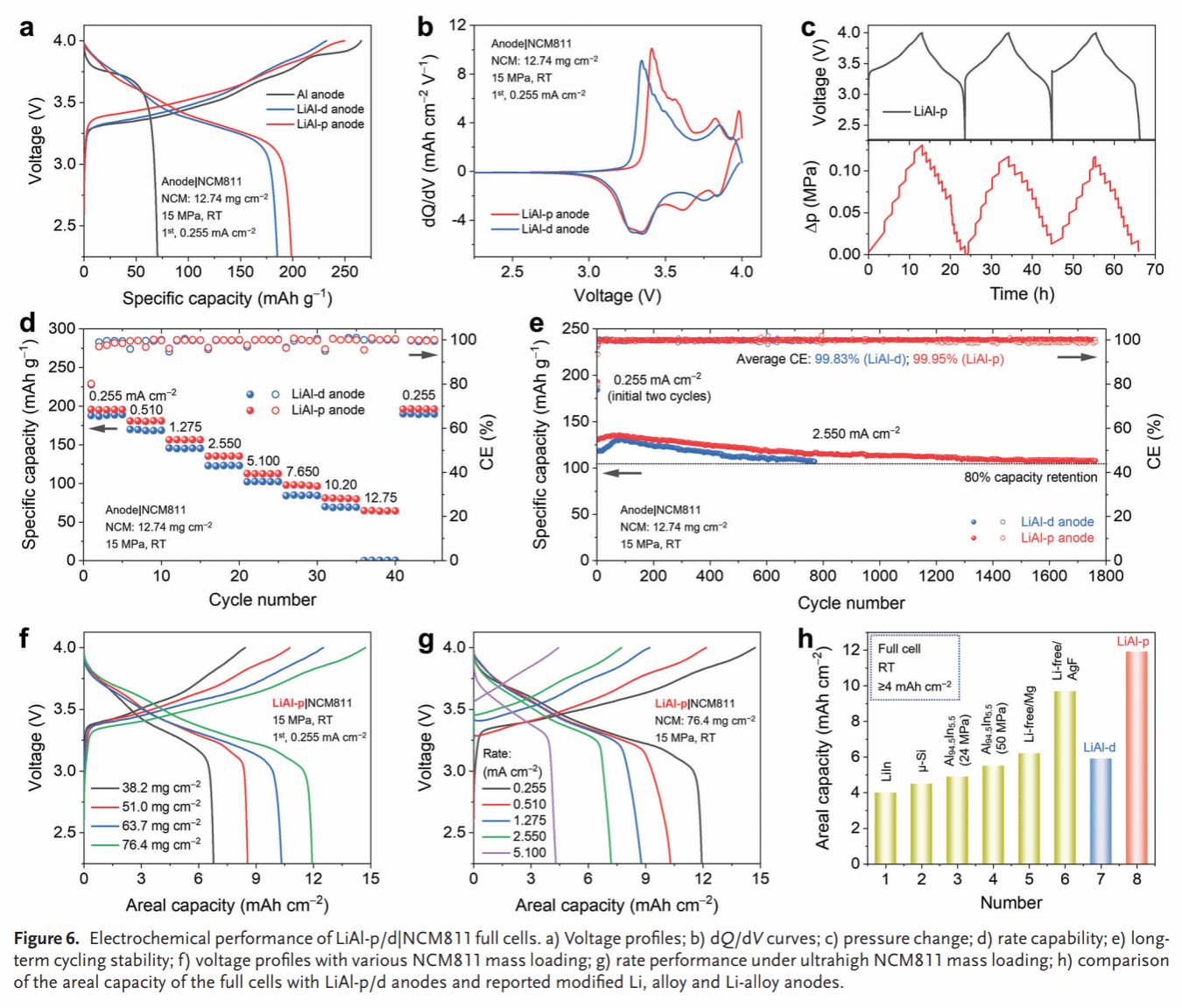
Figure 6. Performance characterization of the LiAl-p/d|NCM811 full cell: charge-discharge curves (a), dQ/dV curves (b), in situ pressure variation curves (c), rate curves (d), long-term cycling curves (e), high-loading charge-discharge curves for the cathode (f, g), and comparison with literature values (h).
The corresponding authors of this paper are Professor Xiaodong Zhuang from Shanghai Jiao Tong University and Professor Xi Zhang from the School of Mechanical and Power Engineering. The first author is Dr. Jinhui Zhu, Assistant Researcher at the Center for Innovation in Synthetic Science, Zhangjiang Advanced Research Institute, and the School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University.
Paper link: https://doi.org/10.1002/adma.202407128
This work was supported by the National Natural Science Foundation of China (NSFC), the Shanghai Municipal Science and Technology Commission, and GF funding.
Contributing Unit:
Center for Innovation in Synthetic Science






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn