搜索


Carbon dioxide (CO₂), as an abundant, non-toxic, and sustainable carbon resource, has drawn extensive attention from chemists for its efficient conversion into various high-value-added chemicals. Over the past decades, researchers have developed a series of methods to convert CO₂ into various carboxylic acid compounds. However, due to CO₂'s inherent thermodynamic stability and kinetic inertness, its conversion typically requires harsh conditions. Furthermore, CO₂'s nonpolar molecular structure results in weak coordination ability with metals. Therefore, synthesizing chiral carboxylic acids from CO₂ via metal-catalyzed asymmetric carbon-carbon bond formation remains a challenging problem in this field. Although some reports on asymmetric catalytic CO₂ transformations have emerged in recent years, most focus on constructing core chirality, while studies utilizing asymmetric carboxylation of CO₂ to build axial chiral carbonyl compounds remain scarce. Over the past decade, synergistic catalytic systems combining photoredox and transition metal catalysis have garnered increasing attention. Compared to single-catalyst systems, this synergistic approach integrates the advantages of photocatalysis and transition metal catalysis. It not only disrupts traditional metal-catalyzed reaction patterns but also introduces greater flexibility in adjusting the valence states of catalytic metals through photocatalysis, thereby enabling novel reaction performance. Furthermore, the independent catalytic cycles of photocatalysis and metal catalysis enable more precise control over reactivity and selectivity.
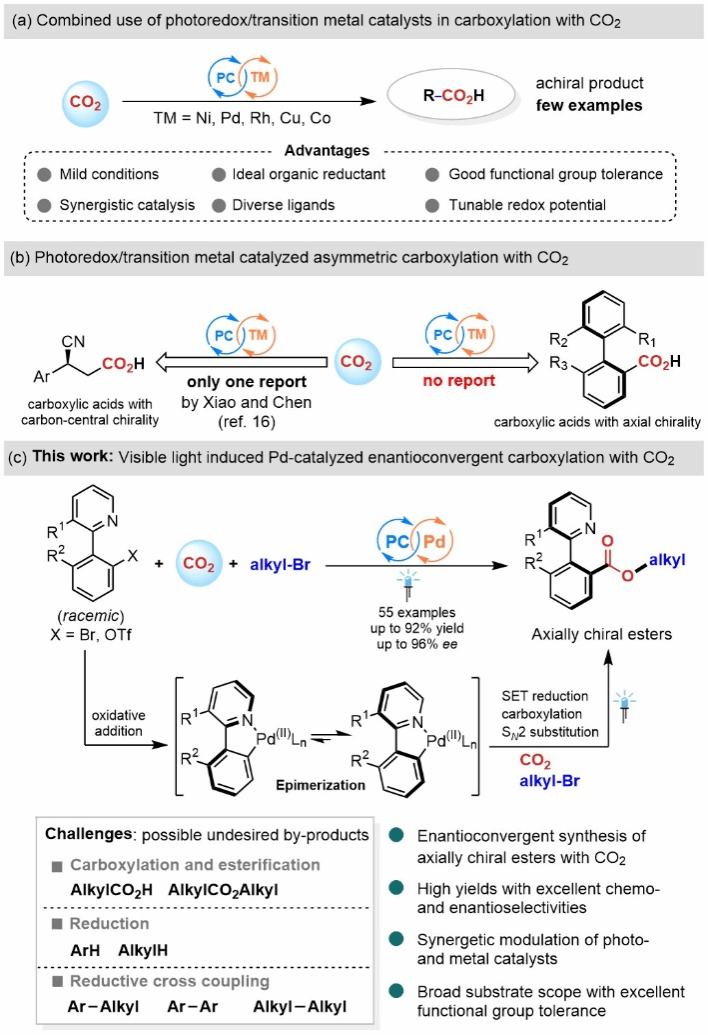
Figure 1. Asymmetric Carboxylation of CO₂ via Photo-Redox/Transition Metal Synergistic Catalysis
Recently, the research group led by Jiawang Liu, Associate Professor on Tenure Track at the Center for Frontier Science in Transformative Molecules, Shanghai Jiao Tong University, and Resident Scientist at the Zhangjiang Advanced Research Institute, developed a tandem asymmetric carboxylation and in situ esterification reaction between heteroaromatic (or halogenated) compounds, CO₂, and alkyl bromides. This was achieved using a palladium-coordinated catalytic system. palladium synergistic catalytic system to develop a tandem asymmetric carboxylation and in situ esterification reaction of fused heteroaromatic (like) halides with CO₂ and alkyl bromides. Through a dynamic kinetic asymmetric transformation process, the team successfully achieved the efficient and highly selective synthesis of axial-chiral esters involving CO₂ (Figure 1). Mechanistic studies reveal that the addition of alkyl bromides rapidly captures the chiral carboxylate anion intermediate in situ, leading to the formation of conformationally stable axial-chiral esters—a critical factor for reaction success.
Using 1-(2-bromonaphthalen-1-yl)isoquinoline 1a and 1-bromooctane 2a as template substrates, 4CzIPN as the photocatalyst, and Pd(acac)₂ as the palladium catalyst precursor, (R)-BTFM-Garphos (L1) as the chiral ligand, DIPEA as the reducing agent, cesium carbonate as the base, lithium bis(trifluoromethanesulfonyl)imide and 4Å molecular sieve as reaction additives, and N, N-dimethylacetamide as the solvent. Under atmospheric CO₂ atmosphere and 455 nm blue light irradiation at 16 °C for 24 hours, product 3aa was obtained with 89% isolated yield and 96% ee. Under optimized conditions, the authors first investigated the scope of alkyl bromide substrates. Various functional groups on multiple alkyl bromides, such as fluorine, chlorine, methoxy, trifluoromethyl, phosphonate groups, and certain drug molecular fragments, demonstrated excellent compatibility (Figure 2).
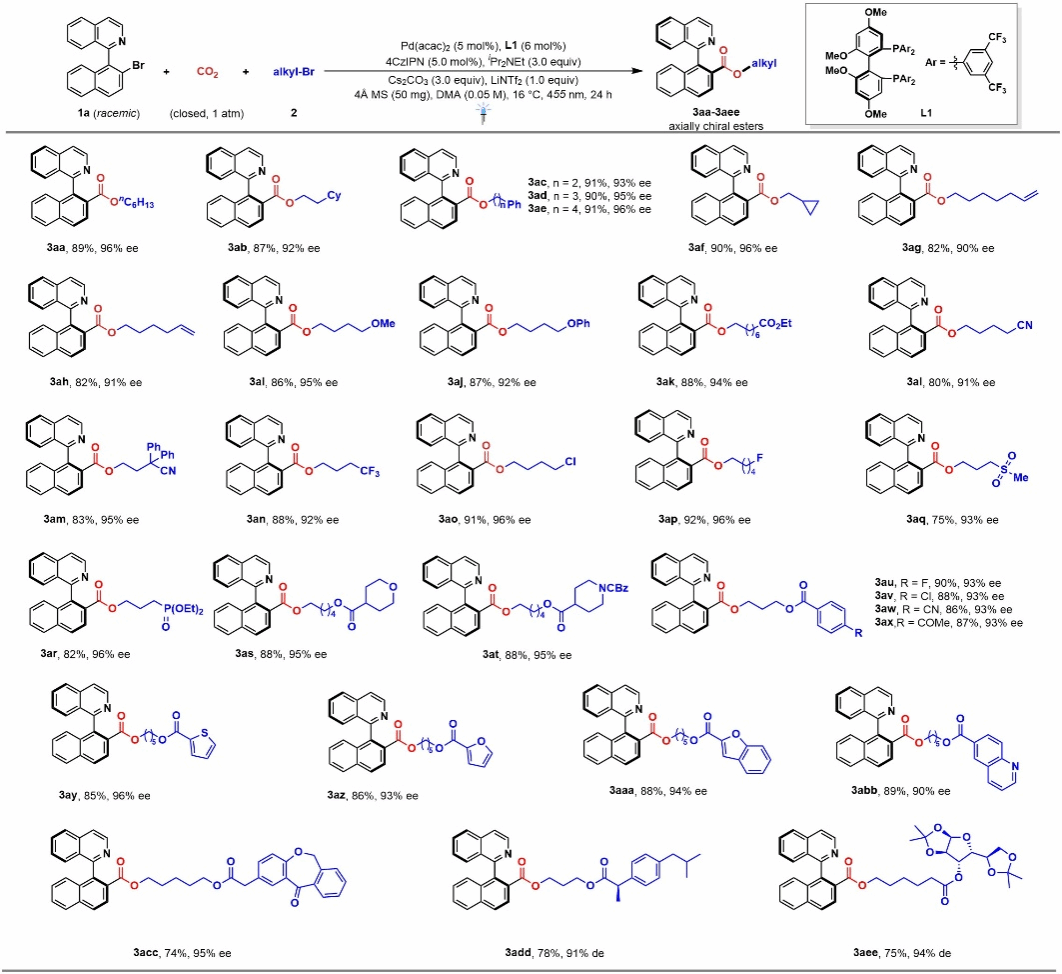
Figure 2 Expansion of Alkyl Bromide Substrates
Subsequently, the authors also investigated dihalogenated heterocyclic compounds. Substituents at various positions on the naphthalene ring had minimal impact on the reaction, yielding the corresponding products in good to excellent yields with outstanding enantioselectivity (Figure 3).
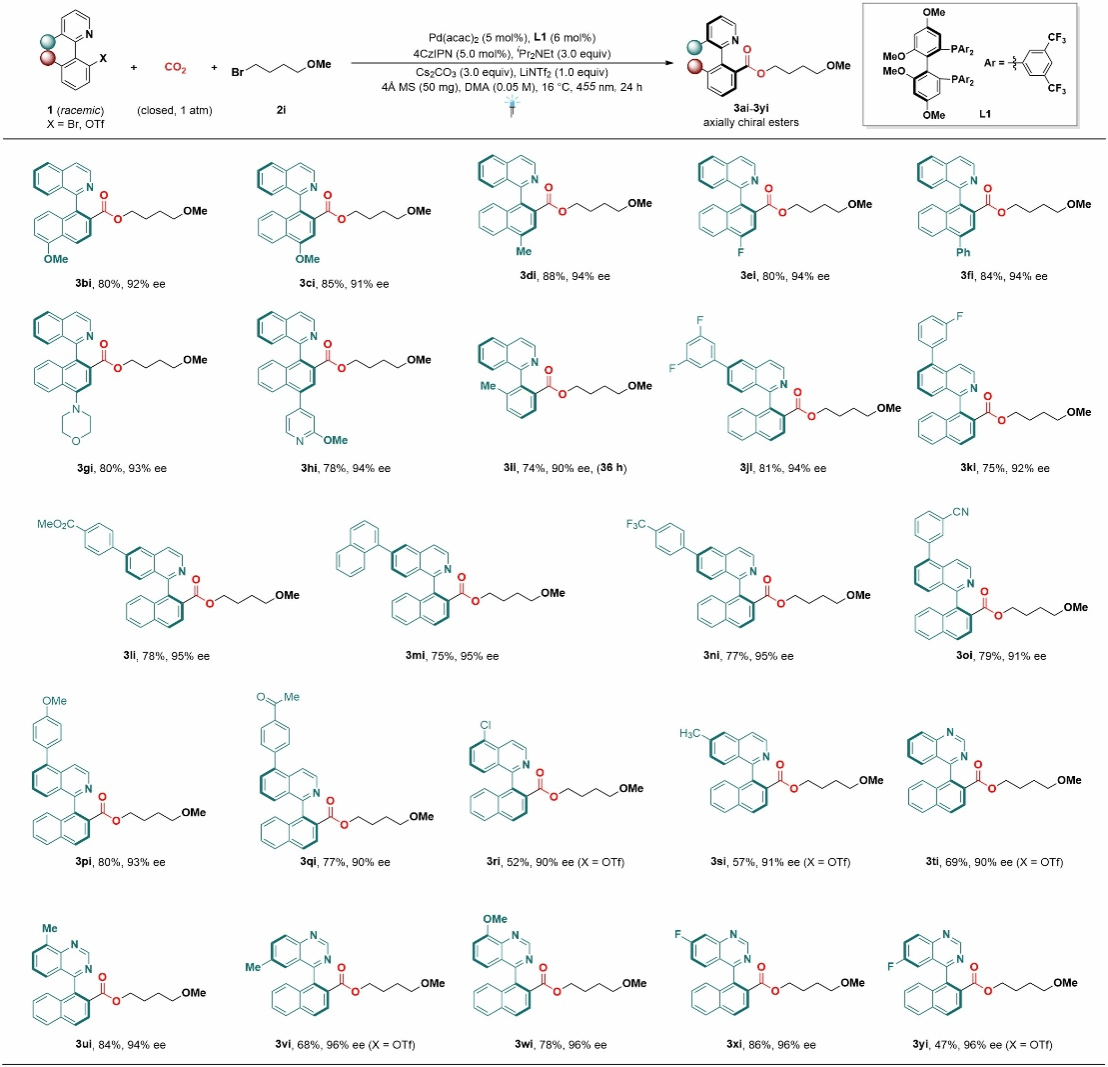
Figure 3 Expansion of Substrate Scope to Halogenated Substances with Conjugated Aromatic Rings
To gain deeper insights into the reaction mechanism, the authors designed a series of control experiments (Figure 4), including: fluorescence quenching studies, cyclic voltammetry, radical quenching and radical clock experiments, reaction kinetics investigations, and product racemization kinetics studies. Experimental results indicate: (1) The reaction involves a tandem enantiomerically convergent carboxylation/substitution esterification process catalyzed by photo-redox/transition metal synergy; (2) The carboxylate anion generated during carboxylation is rapidly captured in situ by alkyl bromide, yielding a conformationally stable axial chiral ester—a key factor contributing to the reaction's high enantioselectivity.
In summary, this work developed an efficient photo-redox/palladium co-catalytic system for synthesizing axial-chiral esters with CO₂ participation. The reaction exhibits high enantioselectivity, broad substrate scope, and strong functional group tolerance. The success hinges on the rapid in situ capture of the chiral carboxylate anion by alkyl bromide, yielding a conformationally stable axial-chiral ester. This reaction not only provides a concise, mild route for synthesizing axial-chiral carboxylate esters but also offers novel insights into the asymmetric conversion of carbon dioxide.
This research was recently published in Angew. Chem. Int. Ed. under the title “Synergistic Photoredox/Palladium Catalyzed Enantioconvergent Carboxylation of Racemic Heterobiaryl (Pseudo)Halides with CO₂.” The corresponding author is Associate Professor Jiawang Liu from Shanghai Jiao Tong University, and the first author is Ye Bihai, a doctoral candidate at Shanghai Jiao Tong University. This work was supported by the Key Research and Development Program of China, the National Natural Science Foundation of China, the Basic Research Fund for Central Universities, the Jiaotong University 2030 Plan, and the Shanghai Rising Star Program.
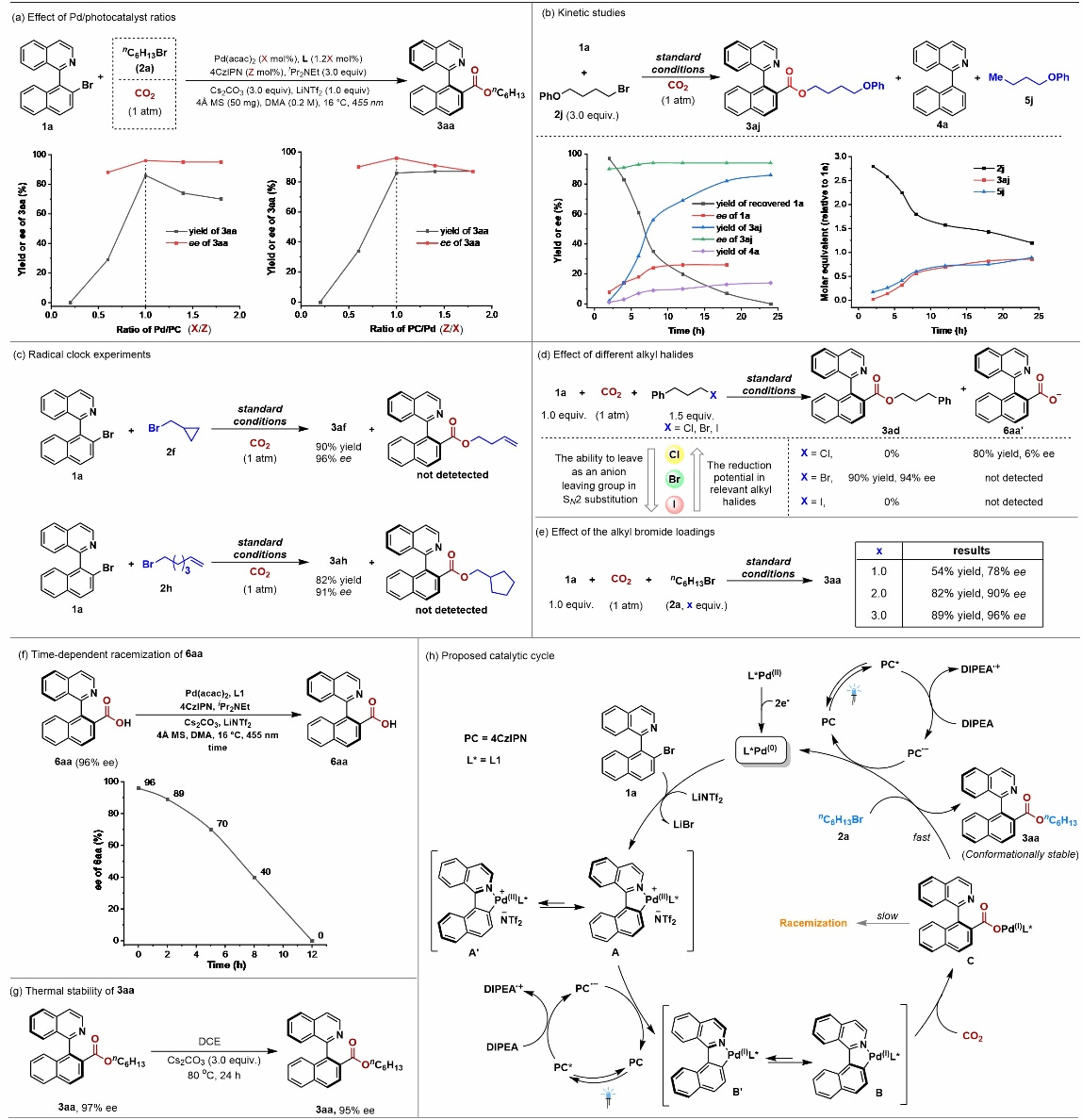
Figure 4: Mechanistic Study
Article link: https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.202413949
Author:
Liu Jiawang Team






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn