搜索


Recently, Nature Communications published online the research paper “Full-course NIR-II imaging-navigated fractionated photodynamic therapy of bladder tumors with X-ray-activated nanotransducers” by the team of Researcher Li Wanwan and Assistant Professor Yu Xujiang (School of Materials Science and Engineering) from the Center for Future Materials Innovation at Shanghai Jiao Tong University's Zhangjiang Advanced Research Institute. The study reports a nanoscintillator based on a Ce³⁺ single-center dual-sensitization mechanism. This nanoscintillator exhibits dual emission in visible and near-infrared II (NIR-II) regions under X-ray irradiation. Combined with a novel imaging system developed by the team, it enables non-invasive quantitative fluorescence imaging analysis and customizable radiodynamic therapy for in situ bladder cancer. The paper received high recognition from editors and was invited to contribute to the “Behind the Paper” section of Nature Portfolio Communities, sharing the research journey.
Bladder cancer is a highly prevalent malignancy among the elderly, with non-muscle-invasive bladder cancer (NMIBC) accounting for over 75% of all cases. Conventional treatments include surgical resection, cystoscopy-assisted photodynamic therapy, and high-dose radiotherapy. Despite significant efficacy, NMIBC patients face substantial risks of recurrence or tumor progression, necessitating long-term follow-up. Consequently, since the 1990s, significant improvements in diagnosis, treatment, and five-year survival rates have remained elusive. This stagnation primarily stems from traditional technologies' inability to integrate real-time diagnosis, synchronized therapy, in vivo monitoring, and long-term prognosis. Thus, developing non-invasive, full-process imaging-guided, and highly effective treatment approaches has become an urgent priority in combating NMIBC.
In recent years, the emergence of “nanomedicine” has significantly elevated cancer treatment through innovative strategies. Pioneering work has been reported, including X-ray-activated photodynamic therapy and X-ray-activated nanomaterials for near-infrared-II imaging. Additionally, antitumor nanodrugs can be locally administered to the bladder via intravesical instillation rather than systemic delivery, ensuring biosafety for clinical translation. Inspired by these advancements, this study integrates photomedicine, X-rays, and nanomedicine to achieve full-disease-course imaging and non-invasive treatment of NMIBC.
Unlike previously reported single-emission nanoscintillators, this study designed a core-shell-shell nanoscintillator structure to achieve synergistic sensitization through multiple luminescence modes (Figure 1). Low-phonon-energy NaGdF₄ was selected as the scintillator matrix. Ce³⁺ and Tb³⁺ were doped into the core, Nd³⁺ into the first shell layer, and the outermost NaLuF₄ layer served as both an inert layer and a sensitization layer. Thermally injected nanocrystals with a core-shell structure were synthesized, spatially isolating doped ions to eliminate cross-relaxation. Ce³⁺ with f-d transitions synergistically sensitized the dual luminescent centers of Tb³⁺ and Nd³⁺, enabling dual emission in the visible and near-infrared II (NIR-II) regions upon X-ray excitation. Results demonstrated that the introduction of Ce³⁺ enhanced visible and NIR-II luminescence by 2.2-fold and 12-fold, respectively.
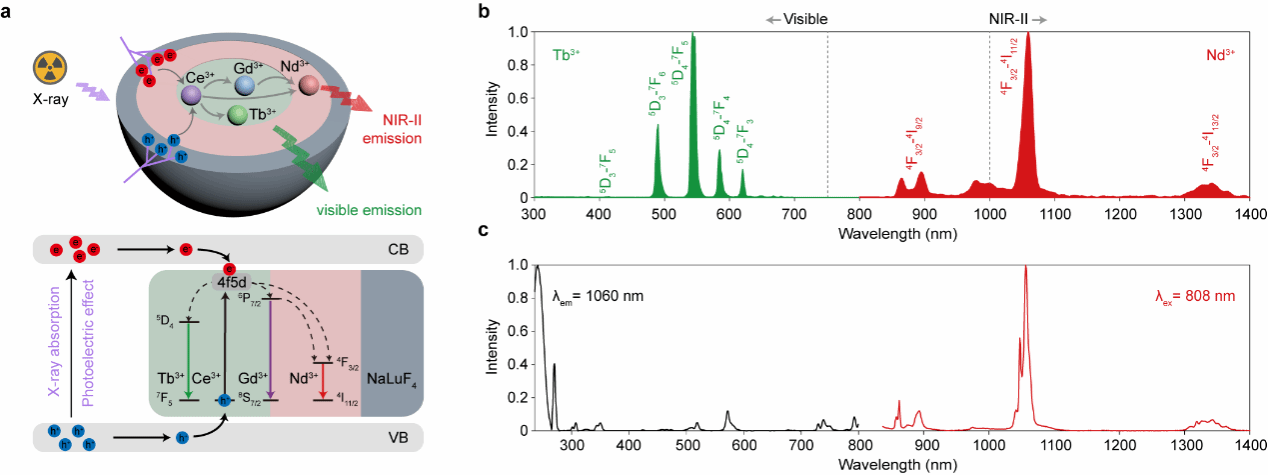
Figure 1. Schematic of the synergistic sensitization mechanism of nanoscintillators and spectral images under X-ray and 808 nm laser irradiation
A nanoscintillator-based diagnostic and therapeutic probe was constructed by functionalizing the surface of nanoscintillators with the photosensitizer Rose Bengal (RB) and the targeting peptide cRGD, achieving reactive oxygen species (ROS) generation and enhancing the probe's tumor enrichment efficiency. In this study, a spontaneous bladder cancer mouse model was established using the drug N-nitroso-N-methylurea (MNU). Histopathological analysis of mouse bladder tissue via H&E staining revealed distinct papillary tumor structures within the bladder, confirming successful model establishment (Figure 2). To validate the specific targeting of tumor tissues by cRGD-modified nanodiagnostic probes, these probes were instilled into the bladders of healthy mice and cancer-bearing mice. As a control, non-cRGD-modified probes were instilled into the bladders of cancer-bearing mice. Ex vivo bladder tissue was analyzed via NIR-II imaging and cryosectioning. Results demonstrated that the cRGD-targeted peptide significantly enhanced the enrichment efficiency of the nanodiagnostic probe within tumor tissues.
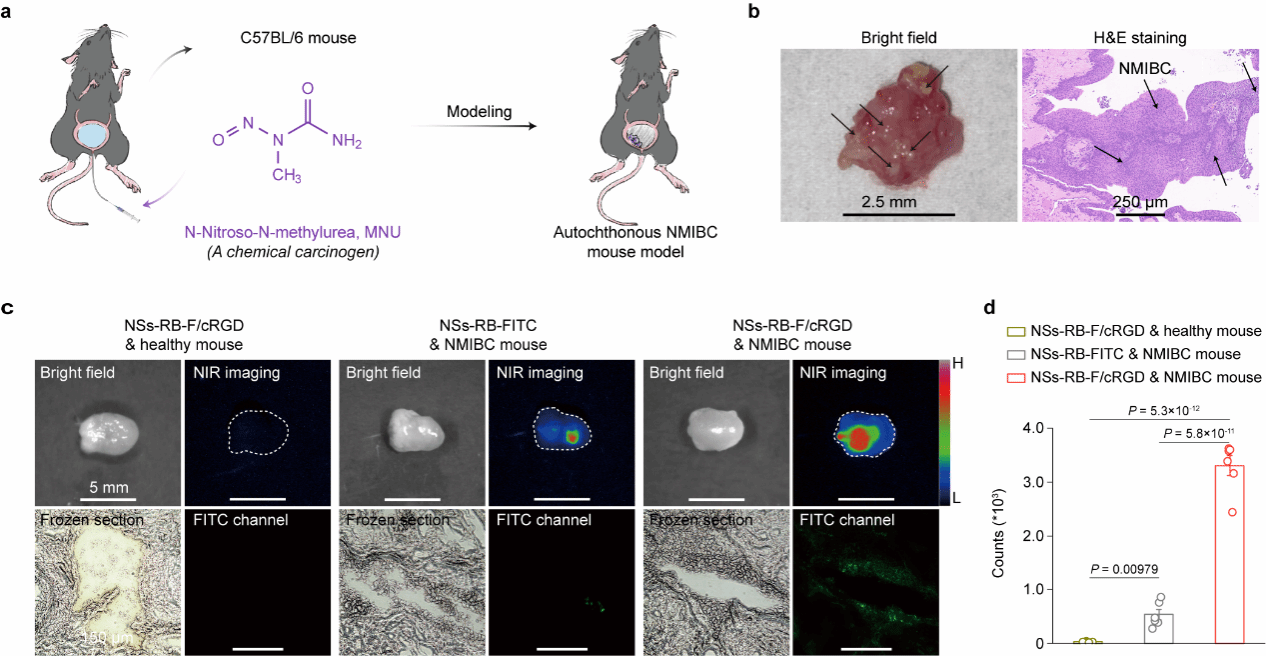
Figure 2 Establishment of Drug-Induced Bladder Cancer Mouse Model and Specific Enrichment of Nanoparticle Theranostic Probe at Tumor Sites
Following the establishment of a spontaneous bladder cancer mouse model, the nanoparticle theranostic probe was instilled into the bladder. After a single 6Gy dose of X-ray irradiation, in vivo NIR-II imaging was performed weekly for five consecutive sessions to monitor disease progression. By monitoring mouse survival rates at 21 and 56 days, combined with H&E staining and immunohistochemical analysis, it was confirmed that a single X-ray treatment could eliminate tumors, improve survival rates, restore immune balance, and suppress tumor recurrence. Furthermore, NIR-II imaging enabled in situ assessment of postoperative tumors (Figure 3).
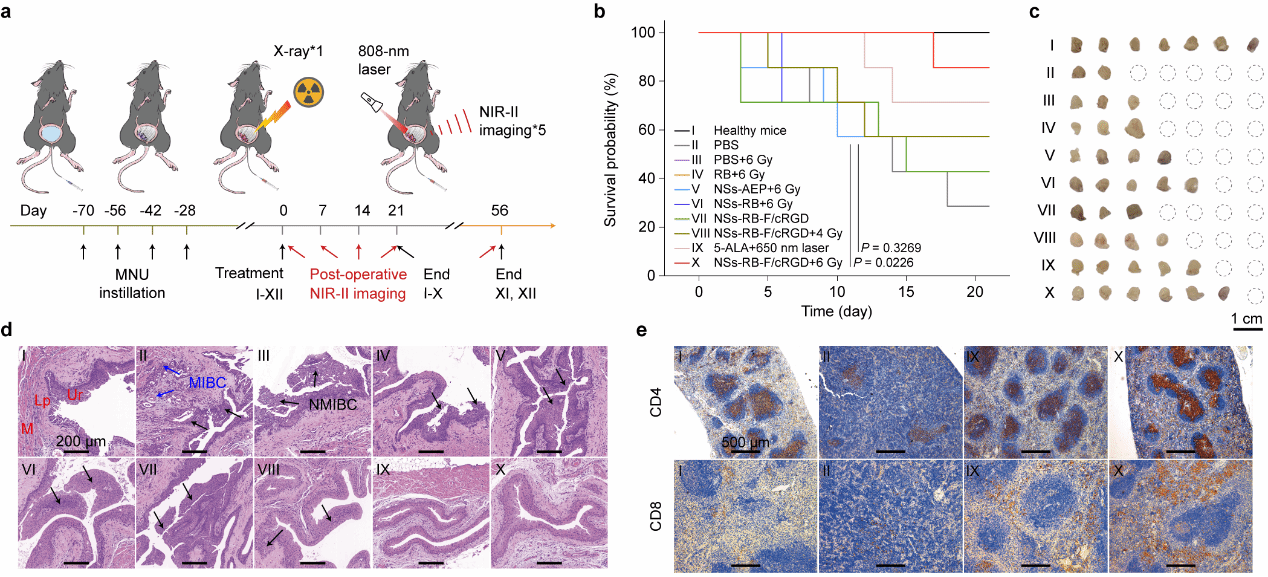
Figure 3: Effective Tumor Elimination Capability Following Single-Session X-Ray-Activated Photodynamic Therapy
The research team further developed a rapid near-infrared photon-counting system capable of multi-source excitation, enabling rapid quantitative analysis of the signal-to-background ratio in imaging. This allows for real-time adjustment of radiation doses (Figure 4). Two distinct radiation experimental groups (G1 and G2) were established. Groups G1 and G2 initially received doses of 3 Gy and 1 Gy, respectively, followed by additional radiation doses based on the signal-to-background ratio from imaging. Group G1 achieved a 56-day survival rate of 100%, while Group G2 reached 80%, indicating the differential impact of varying radiation regimens on tumor treatment and survival rates in mice. Combined with H&E staining and immunohistochemical results from mouse bladder and spleen tissues, this study confirms that NIR-II imaging-guided fractionated photodynamic therapy enables comprehensive preoperative, intraoperative, and postoperative monitoring and decision-making for bladder cancer.
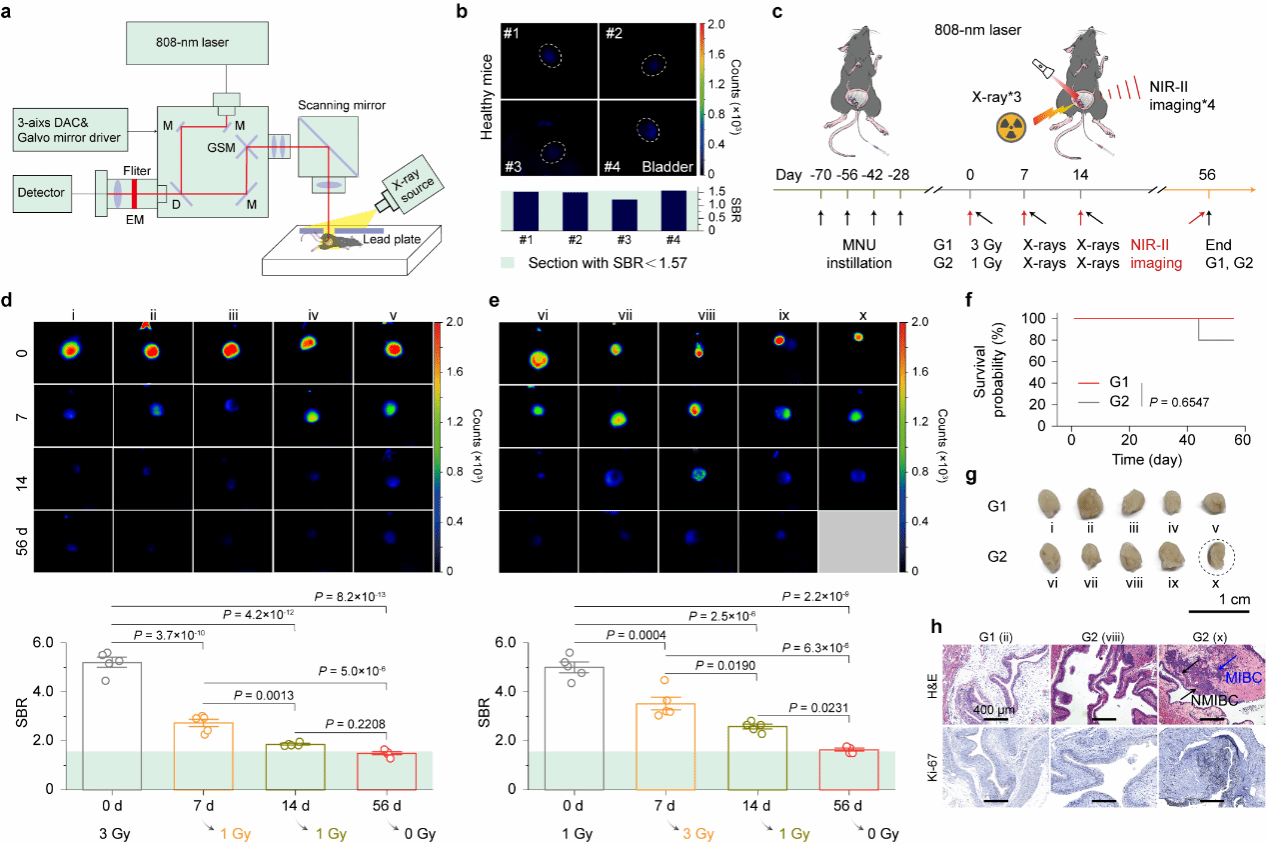
Figure 4 Real-time near-infrared II imaging throughout the process enables on-demand segmentation for photodynamic therapy
This study addresses the current lack of X-ray-excited near-infrared imaging nanoscintillators and the challenges in bladder cancer diagnosis, treatment, and prognosis. It develops a promising nanoprobe and non-invasive, full-process imaging-guided on-demand treatment strategy, demonstrating significant scientific value.
He Liangrui, a doctoral candidate from the School of Materials Science and Engineering at Shanghai Jiao Tong University, and Wang Liyang, a doctoral candidate from the Department of Urology at Renji Hospital affiliated with Shanghai Jiao Tong University School of Medicine, are the co-first authors of the paper. The corresponding authors are Li Wanwan, Researcher, and Yu Xujiang, Assistant Professor, both from the School of Materials Science and Engineering at Shanghai Jiao Tong University; Associate Chief Physician Yang Guoliang from the Department of Urology at Renji Hospital, Shanghai Jiao Tong University School of Medicine, and Professor Liu Zhuang from the Functional Nano and Soft Matter Research Institute (FUNSOM), Soochow University, served as co-corresponding authors. This research was supported by the National Natural Science Foundation of China (82372089, 82272823, 82273288), the National Key R&D Program (2017YFA0205304), the Shanghai Translational Medicine Research Fund (TMSK-2021-117), and the Shanghai Natural Science Foundation (23ZR1434600).
Paper link: https://www.nature.com/articles/s41467-024-52607-9
“Behind the Paper” Research Experience Sharing Link: https://communities.springernature.com/posts/a-promising-nanotechnology-to-unlocking-bladder-cancer-treatment
Author:
He Liangrui, Li Wanwan Team
Contributing Unit:
Center for Future Materials Innovation






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn