搜索


Recently, the internationally renowned materials journal Angewandte Chemie published online the latest research findings from Professor Zhuang Xiaodong's team at the Centre for Innovation in Synthetic Science, Zhangjiang Advanced Research Institute. Titled ‘Two-Dimensional Silver–Isocyanide Frameworks’, the study reports a novel two-dimensional metal-organic framework based on metal-carbon coordination.

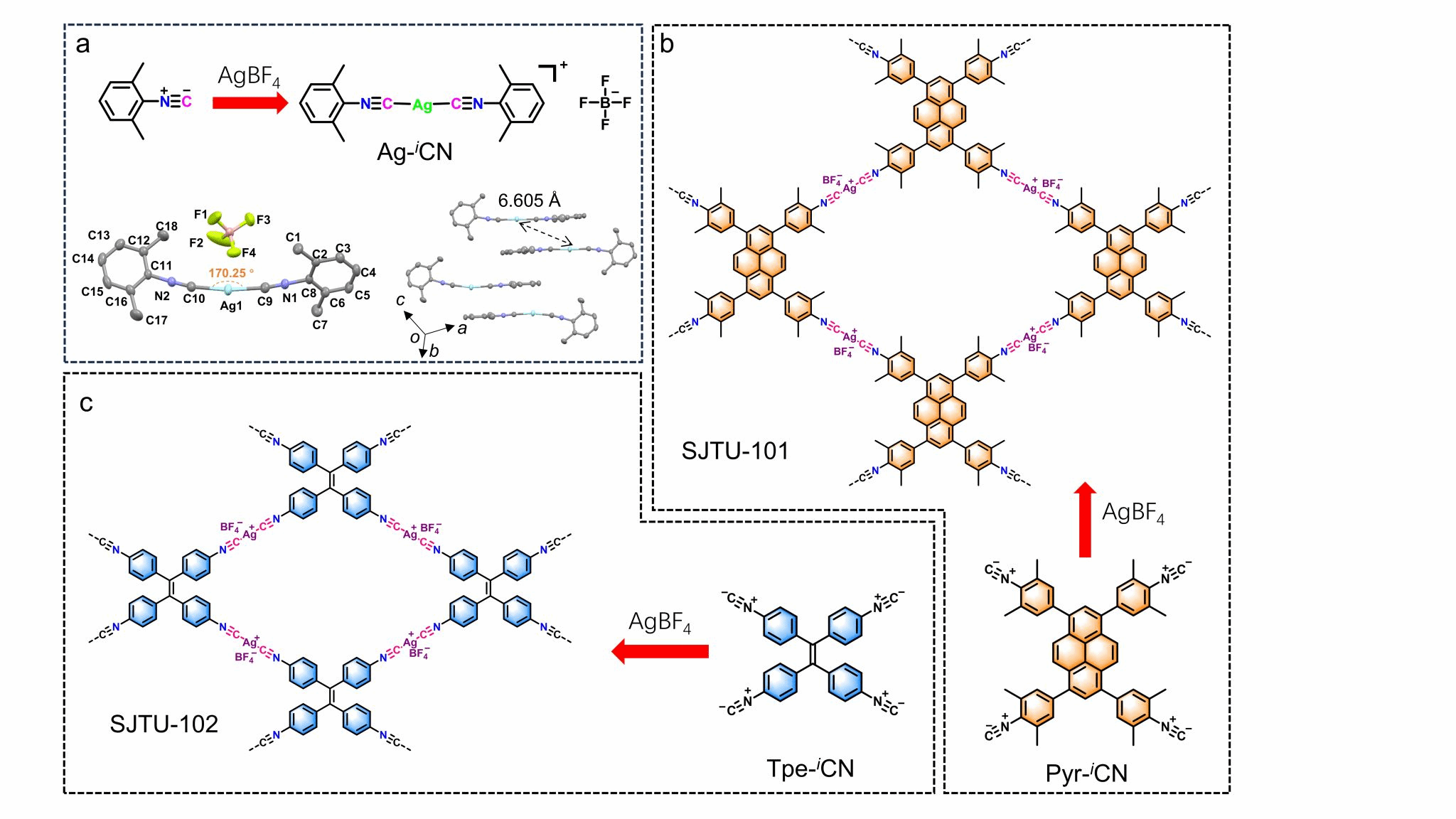
Figure 1. Novel two-dimensional MOFs based on isocyanide–silver coordination. (a) Synthesis of Ag-iCN (lower panel: molecular structure of Ag-iCN (left), crystal stacking distribution of Ag-iCN molecules (right)). Synthesis of SJTU-101 (b) and SJTU-102 (c).
Metal-organic frameworks (MOFs) have been extensively studied due to their versatile applications and readily tunable structures that remain stable in air. Based on hard-soft acid-base theory, MOFs can be modularly synthesised by selecting appropriate metal ions or clusters and organic linkers. Strategies for synthesising MOFs typically involve coordination with metal and heteroatoms, such as O (e.g., hydroxyl, carboxyl), S (e.g., thiol), N (e.g., pyridine, pyrazine), and multiple heteroatoms (e.g., pyrazine with cyano). In contrast, two-dimensional MOFs based on metal–carbon coordination are rarely reported, as most metal–carbon bonds are unstable in aqueous oxygen environments. Although well-defined metal–alkyne-based two-dimensional (2D) coordination has been successfully synthesised on metal surfaces under inert conditions, the demanding preparation conditions limit the widespread application of metal–carbon-based MOFs. The synthesis of MOFs based on metal–carbon coordination remains a challenge.
The novel two-dimensional MOFs developed by Professor Zhuang Xiaodong's team (Figure 1: SJTU-101, SJTU-102) exhibit distinct 020 peaks at 7.69° and 10.74° (2θ) in their WAXS results. The interleaved AB stacking model aligns remarkably well with the Pawley-refined experimental data (Figure 2). Moreover, BF₄⁻ anions are not randomly distributed within the framework space but are closely adjacent to Ag⁺ ions, consistent with their positions in the crystal structure of the model molecule (Ag-iCN, Figure 1a). This highly crystalline structure with a triclinic arrangement and AB stacking pattern indicates that electrostatic interactions between ionic groups dominate over π-π stacking interactions during the reaction process. Moreover, the MOF based on tetraphenylethylene monomers exhibits greater inclination along all three axes compared to the pyrene-based MOF. Aberration-corrected HRTEM images of SJTU-101 reveal a tetragonal array, with bright spots attributed to silver atoms (Figures 3a, 3b), perfectly matching the silver atom arrangement observed in simulated TEM images along the 001 direction. Select area electron diffraction (SAED) patterns along the [001] direction reveal sharp and symmetrical diffraction spots (Fig. 3d, 3g), indicating high crystallinity in both MOFs.
The semiconductor properties of the SJTUs were investigated by combining UV-vis spectroscopy with density functional theory (DFT) calculations, revealing a low optical bandgap of 1.42 eV for SJTU-101 (Figure 4). Employing the synthesised two-dimensional MOFs as catalysts for electrochemical CO₂ reduction, SJTU-101 was found to maintain over 92% Faraday efficiency for CO over a broad potential range of −1.3 to −1.7 V. Theoretical calculations and in situ characterisation suggest that the carbon atom in the isocyanide group may exhibit more pronounced catalytic activity compared to silver sites (Figure 5).
This work has been published online in Angewandte Chemie International Edition (Angew. Chem. Int. Ed. 2024, e202417658) under the title ‘Two-Dimensional Silver–Isocyanide Frameworks’. The first author is Kaiyue Jiang, a doctoral candidate at the School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University.
This research received funding from the National Natural Science Foundation of China, the Shanghai Municipal Science and Technology Commission, and the China Postdoctoral Science Foundation.
Original link:https://doi.org/10.1002/anie.202417658
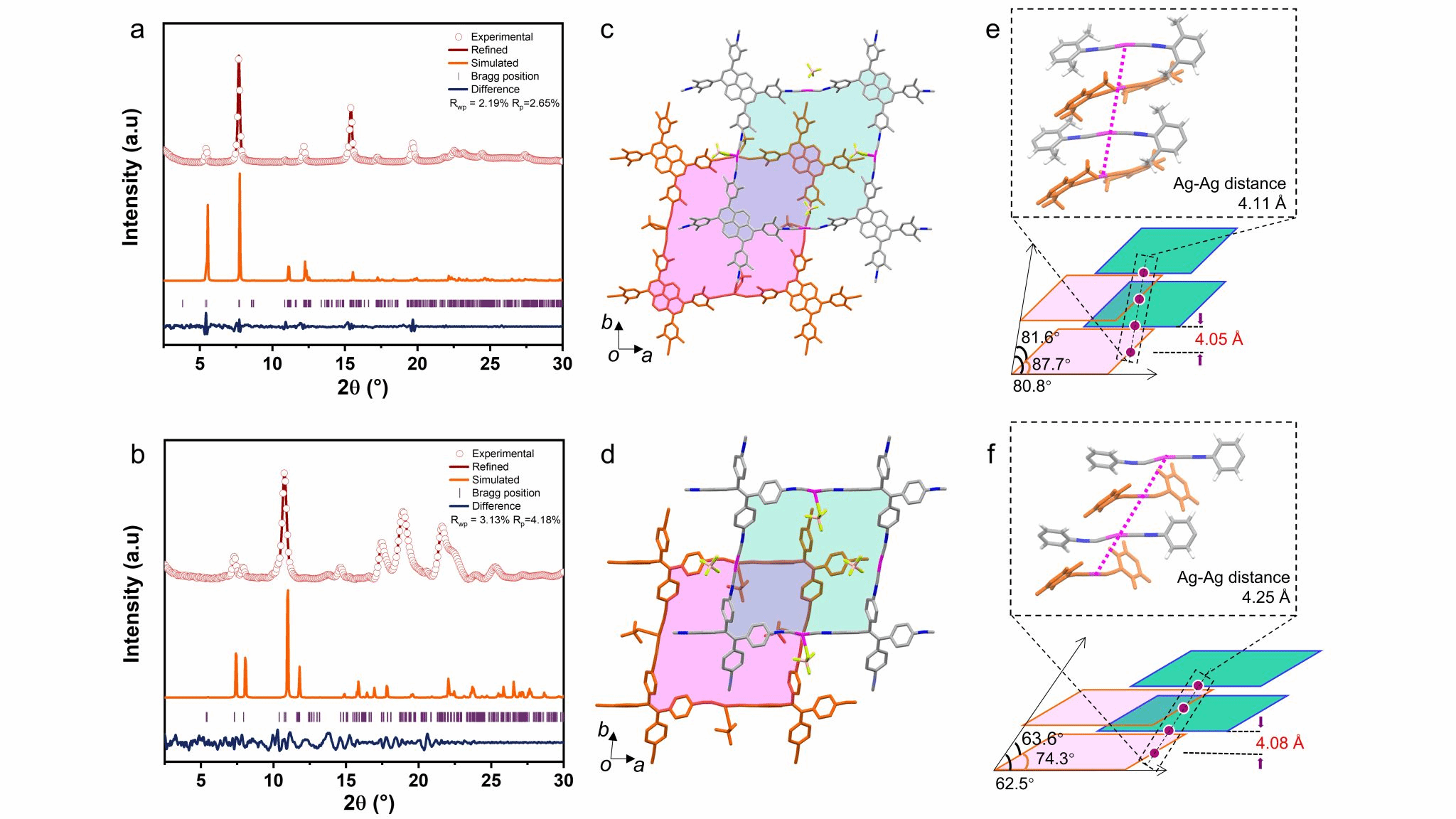
Figure 2. WAXS results for SJTU-101 (a, c) and SJTU-102 (b, d) alongside top-down views of their AB stacking topologies along the c-axis within a 2×2 grid. Schematic diagrams of the stacking structures for SJTU-101 (e) and SJTU-102 (f), along with their unit cell parameters, interlayer spacing, and Ag–Ag distances.
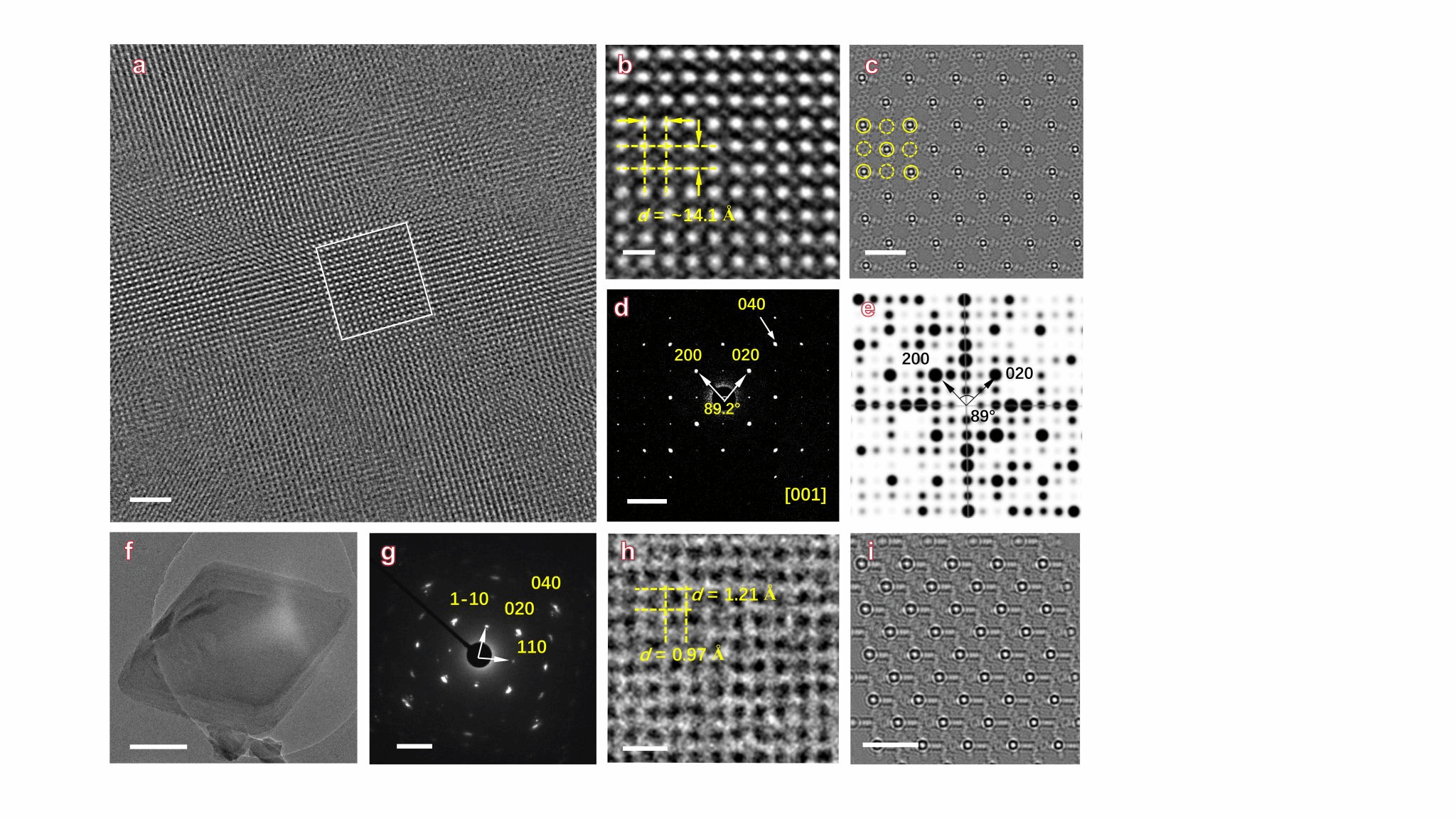
Figure 3. (a) Aberration-corrected HRTEM image of SJTU-101 along the [001] direction. (b) High-magnification micrograph of a selected region in (a). (c) Simulated projection potential map of the optimised structure of SJTU-101 along the [001] direction. (d) SAED pattern and (e) simulated reciprocal lattice image of SJTU-101 along the [001] direction. (f) TEM image, (g) SAED pattern of SJTU-102 along the [001] direction. (h) Filtered TEM image of SJTU-102 and (i) simulated TEM image. Scale bars: (a, b, c, f, h, i) 10 nm, 2 nm, 2 nm, 500 nm, 2 nm, 2 nm; (d, g) 1 nm^(−1).
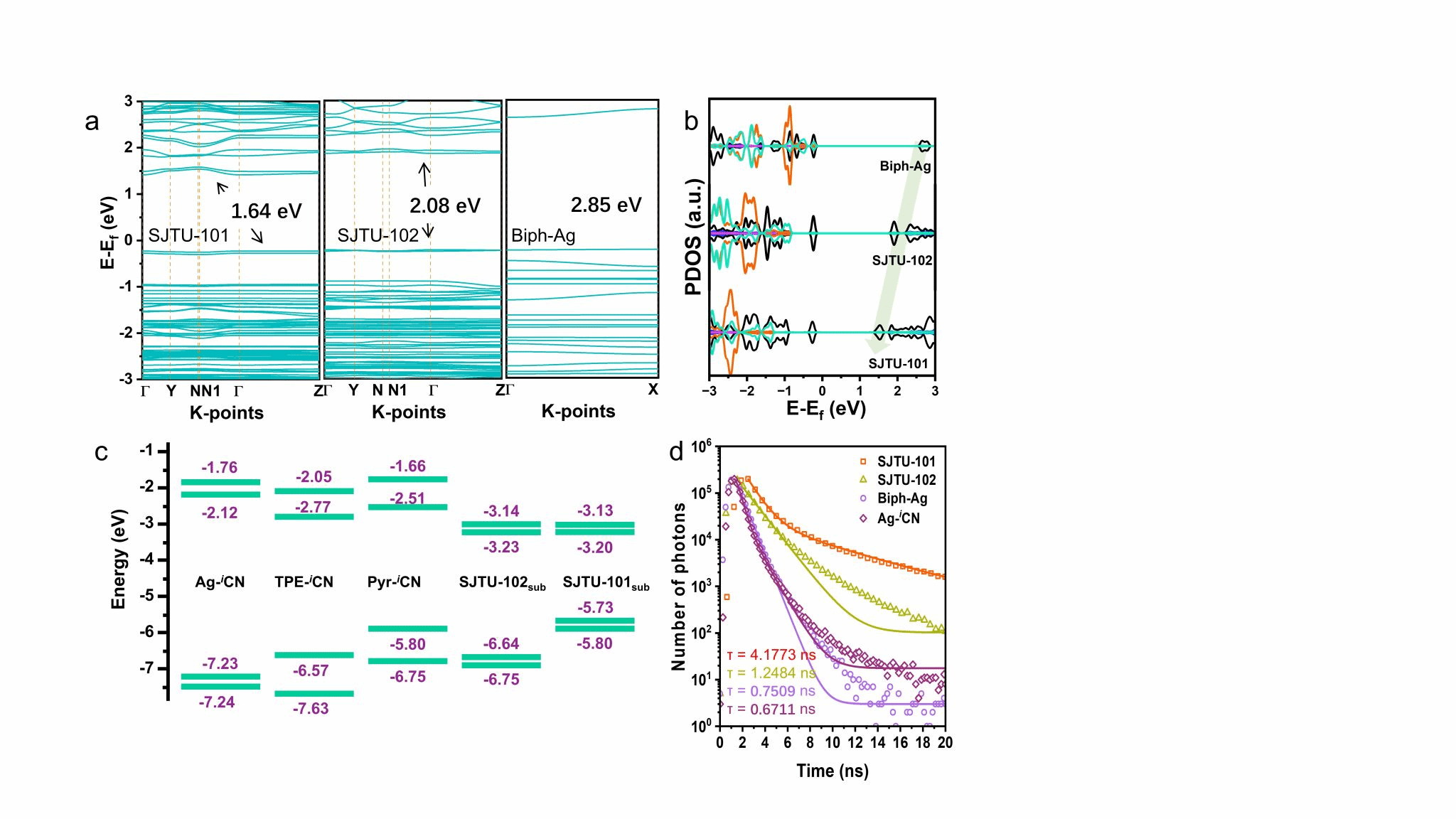
Figure 4. (a) Calculated band structures for Biph-Ag, SJTU-101, and SJTU-102. (b) Partial density of states (PDOS) plots for Biph-Ag (linear), SJTU-101, and SJTU-102, with the Fermi level (0 eV) aligned, showing distinct contributions from C, N, B, F, and Ag (black, blue, pink, orange, and cyan lines respectively) along high-symmetry directions. (c) Level distribution diagrams for Ag-iCN, Tpe-iCN, Pyr-iCN, SJTU-102sub, and SJTU-101sub. The numbers above each structure denote the LUMO/LUMO+1 levels, while those below indicate the HOMO/HOMO-1 levels. (d) Time-resolved fluorescence signal plots for Ag-iCN, Biph-Ag, SJTU-101, and SJTU-102.
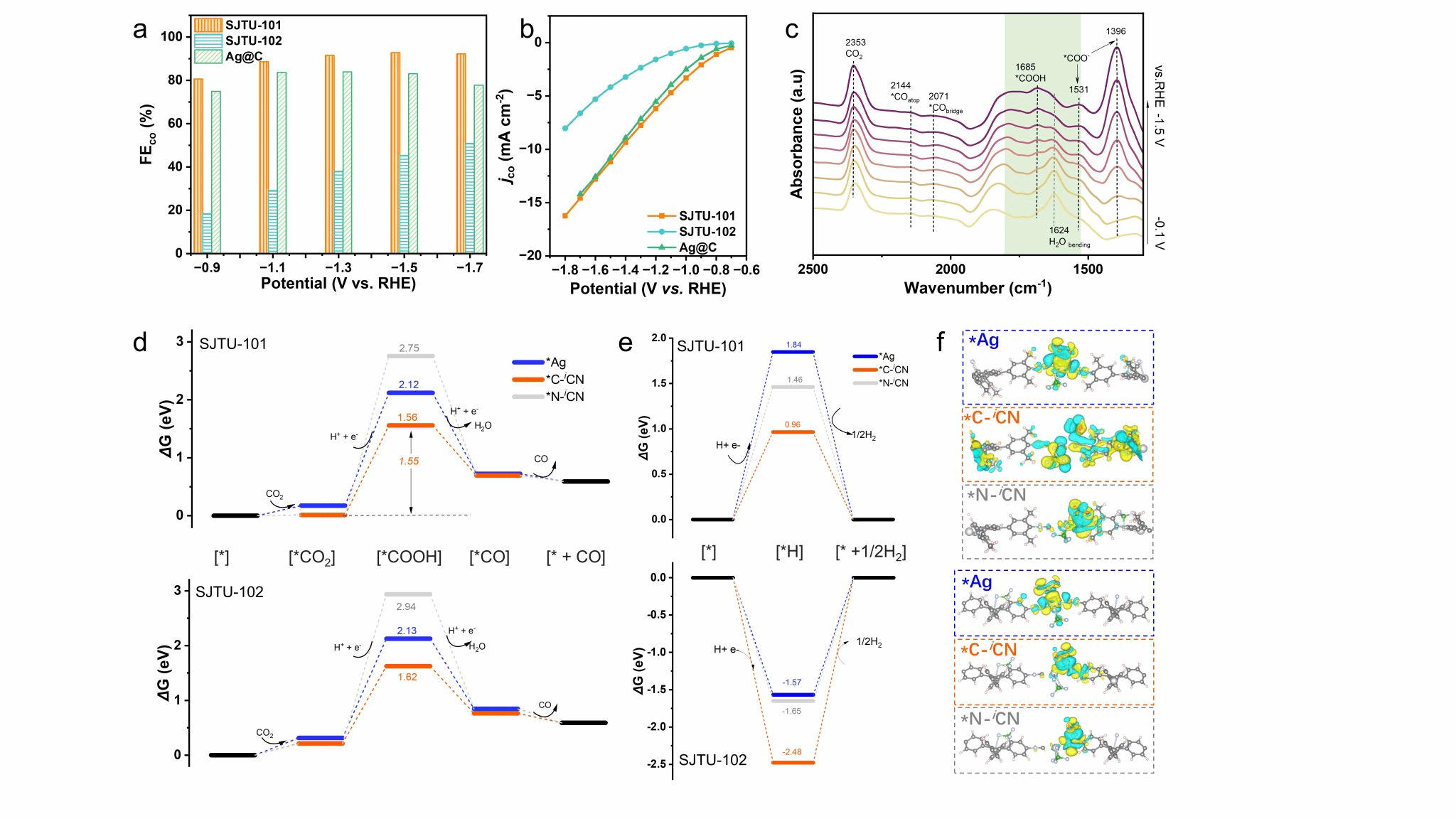
Figure 5. (a) Faradaic efficiency of SJTU-101, SJTU-102 and Ag@C catalysed CO generation in CO₂-saturated 0.1 M KHCO₃, and (b) current density versus potential (vs. RHE). (c) Real-time in situ ATR-FTIR spectra of intermediates during CO₂RR catalysed by SJTU-101 at room temperature. (d) Gibbs free energy curves for CO production via CO₂RR on SJTU-101 and SJTU-102 at U = 0.0 V. (e) Free energy curves for the HER pathway of SJTU-101 and SJTU-102 at U = 0.0 V. (f) Differential charge density plots of SJTU-101 (top) and SJTU-102 (bottom) when *COOH is adsorbed on Ag, CiCN, and NiCN sites respectively.
Contributing Unit:
Centre for Innovation in Synthetic Science






 Address:No.1308 Keyuan Road, Pudong District, Shanghai
Address:No.1308 Keyuan Road, Pudong District, Shanghai Phone:86-21-54740000
Phone:86-21-54740000 E-mail:zias@sjtu.edu.cn
E-mail:zias@sjtu.edu.cn